Human and mouse tissue-engineered small intestine both demonstrate digestive and absorptive function
- PMID: 25573173
- PMCID: PMC4398842
- DOI: 10.1152/ajpgi.00111.2014
Human and mouse tissue-engineered small intestine both demonstrate digestive and absorptive function
Abstract
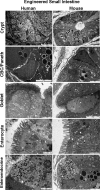
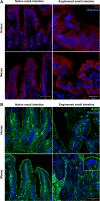
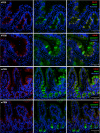
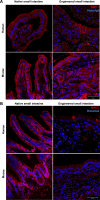
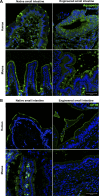
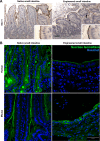
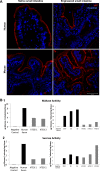
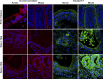
Short bowel syndrome (SBS) is a devastating condition in which insufficient small intestinal surface area results in malnutrition and dependence on intravenous parenteral nutrition. There is an increasing incidence of SBS, particularly in premature babies and newborns with congenital intestinal anomalies. Tissue-engineered small intestine (TESI) offers a therapeutic alternative to the current standard treatment, intestinal transplantation, and has the potential to solve its biggest challenges, namely donor shortage and life-long immunosuppression. We have previously demonstrated that TESI can be generated from mouse and human small intestine and histologically replicates key components of native intestine. We hypothesized that TESI also recapitulates native small intestine function. Organoid units were generated from mouse or human donor intestine and implanted into genetically identical or immunodeficient host mice. After 4 wk, TESI was harvested and either fixed and paraffin embedded or immediately subjected to assays to illustrate function. We demonstrated that both mouse and human tissue-engineered small intestine grew into an appropriately polarized sphere of intact epithelium facing a lumen, contiguous with supporting mesenchyme, muscle, and stem/progenitor cells. The epithelium demonstrated major ultrastructural components, including tight junctions and microvilli, transporters, and functional brush-border and digestive enzymes. This study demonstrates that tissue-engineered small intestine possesses a well-differentiated epithelium with intact ion transporters/channels, functional brush-border enzymes, and similar ultrastructural components to native tissue, including progenitor cells, whether derived from mouse or human cells.
Keywords: intestinal failure; intestinal stem cell; regenerative medicine; short bowel syndrome; tissue engineering.
Copyright © 2015 the American Physiological Society.
Figures



Similar articles
-
Short-term and long-term human or mouse organoid units generate tissue-engineered small intestine without added signalling molecules.Exp Physiol. 2018 Dec;103(12):1633-1644. doi: 10.1113/EP086990. Epub 2018 Nov 2. Exp Physiol. 2018. PMID: 30232817
-
Fgf10 overexpression enhances the formation of tissue-engineered small intestine.J Tissue Eng Regen Med. 2016 Feb;10(2):132-9. doi: 10.1002/term.1720. Epub 2013 Mar 7. J Tissue Eng Regen Med. 2016. PMID: 23468377
-
Vitrification preserves murine and human donor cells for generation of tissue-engineered intestine.J Surg Res. 2014 Aug;190(2):399-406. doi: 10.1016/j.jss.2014.04.041. Epub 2014 May 1. J Surg Res. 2014. PMID: 24857678
-
Tissue engineering the small intestine.Clin Gastroenterol Hepatol. 2013 Apr;11(4):354-8. doi: 10.1016/j.cgh.2013.01.028. Epub 2013 Feb 4. Clin Gastroenterol Hepatol. 2013. PMID: 23380001 Review.
-
Stem cells and biopharmaceuticals: vital roles in the growth of tissue-engineered small intestine.Semin Pediatr Surg. 2014 Jun;23(3):141-9. doi: 10.1053/j.sempedsurg.2014.06.011. Epub 2014 Jun 4. Semin Pediatr Surg. 2014. PMID: 24994528 Review.
Cited by
-
Composite Scaffolds Based on Intestinal Extracellular Matrices and Oxidized Polyvinyl Alcohol: A Preliminary Study for a New Regenerative Approach in Short Bowel Syndrome.Biomed Res Int. 2018 May 27;2018:7824757. doi: 10.1155/2018/7824757. eCollection 2018. Biomed Res Int. 2018. PMID: 29992163 Free PMC article.
-
Successful implantation of an engineered tubular neuromuscular tissue composed of human cells and chitosan scaffold.Surgery. 2015 Dec;158(6):1598-608. doi: 10.1016/j.surg.2015.05.009. Epub 2015 Jun 19. Surgery. 2015. PMID: 26096562 Free PMC article.
-
New approaches to increase intestinal length: Methods used for intestinal regeneration and bioengineering.World J Transplant. 2016 Mar 24;6(1):1-9. doi: 10.5500/wjt.v6.i1.1. World J Transplant. 2016. PMID: 27011901 Free PMC article. Review.
-
Protein-engineered scaffolds for in vitro 3D culture of primary adult intestinal organoids.Biomater Sci. 2015 Oct 15;3(10):1376-85. doi: 10.1039/c5bm00108k. Epub 2015 Jul 16. Biomater Sci. 2015. PMID: 26371971 Free PMC article.
-
Advancing Intestinal Organoid Technology Toward Regenerative Medicine.Cell Mol Gastroenterol Hepatol. 2017 Nov 2;5(1):51-60. doi: 10.1016/j.jcmgh.2017.10.006. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2017. PMID: 29204508 Free PMC article. Review.
References
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. - PubMed
-
- Barthel ER, Levin DE, Speer AL, Sala FG, Torashima Y, Hou X, Grikscheit TC. Human tissue-engineered colon forms from postnatal progenitor cells: an in vivo murine model. Regen Med 7: 807–818, 2012. - PubMed
-
- Cheng H, Merzel J, Leblond CP. Renewal of Paneth cells in the small intestine of the mouse. Am J Anat 126: 507–525, 1969. - PubMed
-
- Choi RS, Riegler M, Pothoulakis C, Kim BS, Mooney D, Vacanti M, Vacanti JP. Studies of brush border enzymes, basement membrane components, and electrophysiology of tissue-engineered neointestine. J Pediatr Surg 33: 991–996; discussion 996–997, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

