Chronic NOS inhibition accelerates NAFLD progression in an obese rat model
- PMID: 25573175
- PMCID: PMC4360049
- DOI: 10.1152/ajpgi.00247.2014
Chronic NOS inhibition accelerates NAFLD progression in an obese rat model
Abstract
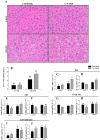
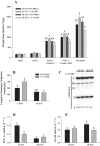
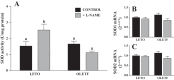
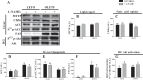
The progression in nonalcoholic fatty liver disease (NAFLD) to nonalcoholic steatohepatitis is a serious health concern, but the underlying mechanisms remain unclear. We hypothesized that chronic inhibition of nitric oxide (NO) synthase (NOS) via N(ω)-nitro-L-arginine methyl ester (L-NAME) would intensify liver injury in a rat model of obesity, insulin resistance, and NAFLD. Obese Otsuka Long-Evans Tokushima fatty (OLETF) and lean Long-Evans Tokushima Otsuka (LETO) rats received control or L-NAME (65-70 mg·kg(-1)·day(-1))-containing drinking water for 4 wk. L-NAME treatment significantly (P < 0.05) reduced serum NO metabolites and food intake in both groups. Remarkably, despite no increase in body weight, L-NAME treatment increased hepatic triacylglycerol content (+40%, P < 0.05) vs. control OLETF rats. This increase was associated with impaired (P < 0.05) hepatic mitochondrial state 3 respiration. Interestingly, the opposite effect was found in LETO rats, where L-NAME increased (P < 0.05) hepatic mitochondrial state 3 respiration. In addition, L-NAME induced a shift toward proinflammatory M1 macrophage polarity, as indicated by elevated hepatic CD11c (P < 0.05) and IL-1β (P = 0.07) mRNA in OLETF rats and reduced expression of the anti-inflammatory M2 markers CD163 and CD206 (P < 0.05) in LETO rats. Markers of total macrophage content (CD68 and F4/80) mRNA were unaffected by L-NAME in either group. In conclusion, systemic NOS inhibition in the obese OLETF rats reduced hepatic mitochondrial respiration, increased hepatic triacylglycerol accumulation, and increased hepatic inflammation. These findings suggest an important role for proper NO metabolism in the hepatic adaptation to obesity.
Keywords: Nω-nitro-l-arginine methyl ester; liver; nitric oxide; obese Otsuka Long-Evans Tokushima fatty rat.
Figures

Similar articles
-
Reduced hepatic eNOS phosphorylation is associated with NAFLD and type 2 diabetes progression and is prevented by daily exercise in hyperphagic OLETF rats.J Appl Physiol (1985). 2014 May 1;116(9):1156-64. doi: 10.1152/japplphysiol.01275.2013. Epub 2014 Feb 27. J Appl Physiol (1985). 2014. PMID: 24577062 Free PMC article.
-
Treating NAFLD in OLETF rats with vigorous-intensity interval exercise training.Med Sci Sports Exerc. 2015 Mar;47(3):556-67. doi: 10.1249/MSS.0000000000000430. Med Sci Sports Exerc. 2015. PMID: 24983336 Free PMC article.
-
Chronic feeding of a nitric oxide synthase inhibitor induces postprandial hypertriglyceridemia in type 2 diabetic model rats, Otsuka Long-Evans Tokushima Fatty rats, but not in nondiabetic rats.Metabolism. 2002 Jun;51(6):702-7. doi: 10.1053/meta.2002.32726. Metabolism. 2002. PMID: 12037722
-
Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model.J Hepatol. 2010 May;52(5):727-36. doi: 10.1016/j.jhep.2009.11.030. Epub 2010 Mar 4. J Hepatol. 2010. PMID: 20347174 Free PMC article.
-
Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model.Am J Physiol Gastrointest Liver Physiol. 2011 May;300(5):G874-83. doi: 10.1152/ajpgi.00510.2010. Epub 2011 Feb 24. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21350190 Free PMC article.
Cited by
-
The Emerging Role of Hepatocellular eNOS in Non-alcoholic Fatty Liver Disease Development.Front Physiol. 2020 Jul 3;11:767. doi: 10.3389/fphys.2020.00767. eCollection 2020. Front Physiol. 2020. PMID: 32719616 Free PMC article. Review.
-
Critical Role for Hepatocyte-Specific eNOS in NAFLD and NASH.Diabetes. 2021 Nov;70(11):2476-2491. doi: 10.2337/db20-1228. Epub 2021 Aug 11. Diabetes. 2021. PMID: 34380696 Free PMC article.
-
Characterization of hepatic fatty acids using magnetic resonance spectroscopy for the assessment of treatment response to metformin in an eNOS-/- mouse model of metabolic nonalcoholic fatty liver disease/nonalcoholic steatohepatitis.NMR Biomed. 2023 Aug;36(8):e4932. doi: 10.1002/nbm.4932. Epub 2023 Apr 11. NMR Biomed. 2023. PMID: 36940044 Free PMC article.
-
Genetics, epigenetics and transgenerational transmission of obesity in children.Front Endocrinol (Lausanne). 2022 Nov 14;13:1006008. doi: 10.3389/fendo.2022.1006008. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36452324 Free PMC article. Review.
-
Ablation of eNOS does not promote adipose tissue inflammation.Am J Physiol Regul Integr Comp Physiol. 2016 Apr 15;310(8):R744-51. doi: 10.1152/ajpregu.00473.2015. Epub 2016 Feb 10. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26864812 Free PMC article.
References
-
- Almeida A, Moncada S, Bolaños JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol 6: 45–51, 2004. - PubMed
-
- Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem 10: 198–206, 1969. - PubMed
-
- Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett 369: 136–139, 1995. - PubMed
-
- Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta 1504: 46–57, 2001. - PubMed
-
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285–289, 1999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

