Chronic NOS inhibition accelerates NAFLD progression in an obese rat model
- PMID: 25573175
- PMCID: PMC4360049
- DOI: 10.1152/ajpgi.00247.2014
Chronic NOS inhibition accelerates NAFLD progression in an obese rat model
Abstract
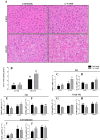
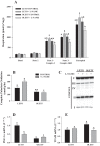
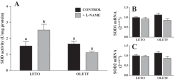
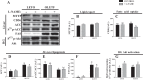
The progression in nonalcoholic fatty liver disease (NAFLD) to nonalcoholic steatohepatitis is a serious health concern, but the underlying mechanisms remain unclear. We hypothesized that chronic inhibition of nitric oxide (NO) synthase (NOS) via N(ω)-nitro-L-arginine methyl ester (L-NAME) would intensify liver injury in a rat model of obesity, insulin resistance, and NAFLD. Obese Otsuka Long-Evans Tokushima fatty (OLETF) and lean Long-Evans Tokushima Otsuka (LETO) rats received control or L-NAME (65-70 mg·kg(-1)·day(-1))-containing drinking water for 4 wk. L-NAME treatment significantly (P < 0.05) reduced serum NO metabolites and food intake in both groups. Remarkably, despite no increase in body weight, L-NAME treatment increased hepatic triacylglycerol content (+40%, P < 0.05) vs. control OLETF rats. This increase was associated with impaired (P < 0.05) hepatic mitochondrial state 3 respiration. Interestingly, the opposite effect was found in LETO rats, where L-NAME increased (P < 0.05) hepatic mitochondrial state 3 respiration. In addition, L-NAME induced a shift toward proinflammatory M1 macrophage polarity, as indicated by elevated hepatic CD11c (P < 0.05) and IL-1β (P = 0.07) mRNA in OLETF rats and reduced expression of the anti-inflammatory M2 markers CD163 and CD206 (P < 0.05) in LETO rats. Markers of total macrophage content (CD68 and F4/80) mRNA were unaffected by L-NAME in either group. In conclusion, systemic NOS inhibition in the obese OLETF rats reduced hepatic mitochondrial respiration, increased hepatic triacylglycerol accumulation, and increased hepatic inflammation. These findings suggest an important role for proper NO metabolism in the hepatic adaptation to obesity.
Keywords: Nω-nitro-l-arginine methyl ester; liver; nitric oxide; obese Otsuka Long-Evans Tokushima fatty rat.
Figures

References
-
- Almeida A, Moncada S, Bolaños JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol 6: 45–51, 2004. - PubMed
-
- Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem 10: 198–206, 1969. - PubMed
-
- Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett 369: 136–139, 1995. - PubMed
-
- Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta 1504: 46–57, 2001. - PubMed
-
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285–289, 1999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

