NVP-QBE170: an inhaled blocker of the epithelial sodium channel with a reduced potential to induce hyperkalaemia
- PMID: 25573195
- PMCID: PMC4439877
- DOI: 10.1111/bph.13075
NVP-QBE170: an inhaled blocker of the epithelial sodium channel with a reduced potential to induce hyperkalaemia
Abstract
Background and purpose: Inhaled amiloride, a blocker of the epithelial sodium channel (ENaC), enhances mucociliary clearance (MCC) in cystic fibrosis (CF) patients. However, the dose of amiloride is limited by the mechanism-based side effect of hyperkalaemia resulting from renal ENaC blockade. Inhaled ENaC blockers with a reduced potential to induce hyperkalaemia provide a therapeutic strategy to improve mucosal hydration and MCC in the lungs of CF patients. The present study describes the preclinical profile of a novel ENaC blocker, NVP-QBE170, designed for inhaled delivery, with a reduced potential to induce hyperkalaemia.
Experimental approach: The in vitro potency and duration of action of NVP-QBE170 were compared with amiloride and a newer ENaC blocker, P552-02, in primary human bronchial epithelial cells (HBECs) by short-circuit current. In vivo efficacy and safety were assessed in guinea pig (tracheal potential difference/hyperkalaemia), rat (hyperkalaemia) and sheep (MCC).
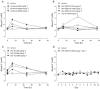
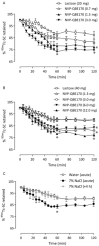
Key results: In vitro, NVP-QBE170 potently inhibited ENaC function in HBEC and showed a longer duration of action to comparator molecules. In vivo, intratracheal (i.t.) instillation of NVP-QBE170 attenuated ENaC activity in the guinea pig airways with greater potency and duration of action than that of amiloride without inducing hyperkalaemia in either guinea pig or rat. Dry powder inhalation of NVP-QBE170 by conscious sheep increased MCC and was better than inhaled hypertonic saline in terms of efficacy and duration of action.
Conclusions and implications: NVP-QBE170 highlights the potential for inhaled ENaC blockers to exhibit efficacy in the airways with a reduced risk of hyperkalaemia, relative to existing compounds.
© 2015 The British Pharmacological Society.
Figures



Similar articles
-
Preclinical evaluation of the epithelial sodium channel inhibitor AZD5634 and implications on human translation.Am J Physiol Lung Cell Mol Physiol. 2022 Nov 1;323(5):L536-L547. doi: 10.1152/ajplung.00454.2021. Epub 2022 Sep 13. Am J Physiol Lung Cell Mol Physiol. 2022. PMID: 36098422 Free PMC article.
-
SPX-101 Is a Novel Epithelial Sodium Channel-targeted Therapeutic for Cystic Fibrosis That Restores Mucus Transport.Am J Respir Crit Care Med. 2017 Sep 15;196(6):734-744. doi: 10.1164/rccm.201612-2445OC. Am J Respir Crit Care Med. 2017. PMID: 28481660
-
ETD001: A novel inhaled ENaC blocker with an extended duration of action in vivo.J Cyst Fibros. 2025 Jan;24(1):72-78. doi: 10.1016/j.jcf.2024.06.002. Epub 2024 Jun 8. J Cyst Fibros. 2025. PMID: 38851923
-
ENaC inhibitors and airway re-hydration in cystic fibrosis: state of the art.Curr Mol Pharmacol. 2013 Mar;6(1):3-12. doi: 10.2174/18744672112059990025. Curr Mol Pharmacol. 2013. PMID: 23547930 Review.
-
Novel small molecule epithelial sodium channel inhibitors as potential therapeutics in cystic fibrosis - a patent evaluation.Expert Opin Ther Pat. 2013 Oct;23(10):1383-9. doi: 10.1517/13543776.2013.829454. Epub 2013 Aug 19. Expert Opin Ther Pat. 2013. PMID: 23957246 Review.
Cited by
-
ENaC inhibition in cystic fibrosis: potential role in the new era of CFTR modulator therapies.Eur Respir J. 2020 Dec 24;56(6):2000946. doi: 10.1183/13993003.00946-2020. Print 2020 Dec. Eur Respir J. 2020. PMID: 32732328 Free PMC article. Review.
-
The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis.Curr Opin Pharmacol. 2018 Dec;43:152-165. doi: 10.1016/j.coph.2018.09.007. Epub 2018 Oct 16. Curr Opin Pharmacol. 2018. PMID: 30340955 Free PMC article. Review.
-
Recent advances in developing therapeutics for cystic fibrosis.Hum Mol Genet. 2018 Aug 1;27(R2):R173-R186. doi: 10.1093/hmg/ddy188. Hum Mol Genet. 2018. PMID: 30060192 Free PMC article. Review.
-
Marked increases in mucociliary clearance produced by synergistic secretory agonists or inhibition of the epithelial sodium channel.Sci Rep. 2016 Nov 10;6:36806. doi: 10.1038/srep36806. Sci Rep. 2016. PMID: 27830759 Free PMC article.
-
The Epithelial Sodium Channel-An Underestimated Drug Target.Int J Mol Sci. 2023 Apr 24;24(9):7775. doi: 10.3390/ijms24097775. Int J Mol Sci. 2023. PMID: 37175488 Free PMC article. Review.
References
-
- App EM, King M, Helfesrieder R, Köhler D, Matthys H. Acute and long-term amiloride inhalation in cystic fibrosis lung disease. A rational approach to cystic fibrosis therapy. Am Rev Respir Dis. 1990;141:605–612. - PubMed
-
- Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261:5–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

