The effects of sigma (σ1) receptor-selective ligands on muscarinic receptor antagonist-induced cognitive deficits in mice
- PMID: 25573298
- PMCID: PMC4409904
- DOI: 10.1111/bph.13076
The effects of sigma (σ1) receptor-selective ligands on muscarinic receptor antagonist-induced cognitive deficits in mice
Abstract
Background and purpose: Cognitive deficits in patients with Alzheimer's disease, Parkinson's disease, traumatic brain injury and stroke often involve alterations in cholinergic signalling. Currently available therapeutic drugs provide only symptomatic relief. Therefore, novel therapeutic strategies are needed to retard and/or arrest the progressive loss of memory.
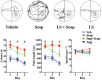
Experimental approach: Scopolamine-induced memory impairment provides a rapid and reversible phenotypic screening paradigm for cognition enhancement drug discovery. Male C57BL/6J mice given scopolamine (1 mg·kg(-1) ) were used to evaluate the ability of LS-1-137, a novel sigma (σ1) receptor-selective agonist, to improve the cognitive deficits associated with muscarinic antagonist administration.
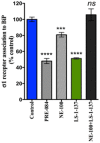
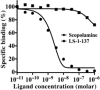
Key results: LS-1-137 is a high-affinity (Ki = 3.2 nM) σ1 receptor agonist that is 80-fold selective for σ1, compared with σ2 receptors. LS-1-137 binds with low affinity at D2-like (D2, D3 and D4) dopamine and muscarinic receptors. LS-1-137 was found to partially reverse the learning deficits associated with scopolamine administration using a water maze test and an active avoidance task. LS-1-137 treatment was also found to trigger the release of brain-derived neurotrophic factor from rat astrocytes.
Conclusions and implications: The σ1 receptor-selective compound LS-1-137 may represent a novel candidate cognitive enhancer for the treatment of muscarinic receptor-dependent cognitive deficits.
© 2015 The British Pharmacological Society.
Figures



Similar articles
-
SA4503, a novel cognitive enhancer with sigma1 receptor agonist properties, facilitates NMDA receptor-dependent learning in mice.Eur J Pharmacol. 1997 Jun 5;328(1):9-18. doi: 10.1016/s0014-2999(97)83020-8. Eur J Pharmacol. 1997. PMID: 9203561
-
AF710B, a Novel M1/σ1 Agonist with Therapeutic Efficacy in Animal Models of Alzheimer’s Disease.Neurodegener Dis. 2016;16(1-2):95-110. doi: 10.1159/000440864. Neurodegener Dis. 2016. PMID: 26606130 Free PMC article.
-
Anti-amnesic properties of (+/-)-PPCC, a novel sigma receptor ligand, on cognitive dysfunction induced by selective cholinergic lesion in rats.J Neurochem. 2009 May;109(3):744-54. doi: 10.1111/j.1471-4159.2009.06000.x. Epub 2009 Feb 20. J Neurochem. 2009. PMID: 19245662
-
The Revival of Scopolamine Reversal for the Assessment of Cognition-Enhancing Drugs.In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 17. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 17. PMID: 21204339 Free Books & Documents. Review.
-
Beyond the blur: Scopolamine's utility and limits in modeling cognitive disorders across sexes - Narrative review.Ageing Res Rev. 2025 Feb;104:102635. doi: 10.1016/j.arr.2024.102635. Epub 2024 Dec 7. Ageing Res Rev. 2025. PMID: 39653154 Review.
Cited by
-
The Roles of Intracellular Chaperone Proteins, Sigma Receptors, in Parkinson's Disease (PD) and Major Depressive Disorder (MDD).Front Pharmacol. 2019 May 21;10:528. doi: 10.3389/fphar.2019.00528. eCollection 2019. Front Pharmacol. 2019. PMID: 31178723 Free PMC article. Review.
-
Sigma-1 Receptor (S1R) Interaction with Cholesterol: Mechanisms of S1R Activation and Its Role in Neurodegenerative Diseases.Int J Mol Sci. 2021 Apr 15;22(8):4082. doi: 10.3390/ijms22084082. Int J Mol Sci. 2021. PMID: 33920913 Free PMC article.
-
Pridopidine activates neuroprotective pathways impaired in Huntington Disease.Hum Mol Genet. 2016 Sep 15;25(18):3975-3987. doi: 10.1093/hmg/ddw238. Epub 2016 Jul 27. Hum Mol Genet. 2016. PMID: 27466197 Free PMC article.
-
Targeting the Sigma-1 Receptor via Pridopidine Ameliorates Central Features of ALS Pathology in a SOD1G93A Model.Cell Death Dis. 2019 Mar 1;10(3):210. doi: 10.1038/s41419-019-1451-2. Cell Death Dis. 2019. PMID: 30824685 Free PMC article.
-
Structural and Molecular Insight into Piperazine and Piperidine Derivatives as Histamine H3 and Sigma-1 Receptor Antagonists with Promising Antinociceptive Properties.ACS Chem Neurosci. 2022 Jan 5;13(1):1-15. doi: 10.1021/acschemneuro.1c00435. Epub 2021 Dec 15. ACS Chem Neurosci. 2022. PMID: 34908391 Free PMC article.
References
-
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013a;170:1449–1458. - PubMed
-
- Antonini V, Prezzavento O, Coradazzi M, Marrazzo A, Ronsisvalle S, Arena E, et al. Anti-amnesic properties of (+/−)-PPCC, a novel sigma receptor ligand, on cognitive dysfunction induced by selective cholinergic lesion in rats. J Neurochem. 2009;109:744–754. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

