Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients
- PMID: 25573405
- PMCID: PMC6669032
- DOI: 10.1183/09031936.00169714
Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients
Abstract
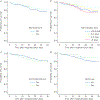
We aimed to study factors influencing outcomes of adults hospitalised for seasonal and pandemic influenza. Individual-patient data from three Asian cohorts (Hong Kong, Singapore and Beijing; N=2649) were analysed. Adults hospitalised for laboratory-confirmed influenza (prospectively diagnosed) during 2008-2011 were studied. The primary outcome measure was 30-day survival. Multivariate Cox regression models (time-fixed and time-dependent) were used. Patients had high morbidity (respiratory/nonrespiratory complications in 68.4%, respiratory failure in 48.6%, pneumonia in 40.8% and bacterial superinfections in 10.8%) and mortality (5.9% at 30 days and 6.9% at 60 days). 75.2% received neuraminidase inhibitors (NAI) (73.8% received oseltamivir and 1.4% received peramivir/zanamivir; 44.5% of patients received NAI ≤2 days and 65.5% ≤5 days after onset of illness); 23.1% received systemic corticosteroids. There were fewer deaths among NAI-treated patients (5.3% versus 7.6%; p=0.032). NAI treatment was independently associated with survival (adjusted hazard ratio (HR) 0.28, 95% CI 0.19-0.43), adjusted for treatment-propensity score and patient characteristics. Superinfections increased (adjusted HR 2.18, 95% CI 1.52-3.11) and chronic statin use decreased (adjusted HR 0.44, 95% CI 0.23-0.84) death risks. Best survival was shown when treatment started within ≤2 days (adjusted HR 0.20, 95% CI 0.12-0.32), but there was benefit with treatment within 3-5 days (adjusted HR 0.35, 95% CI 0.21-0.58). Time-dependent analysis showed consistent results of NAI treatment (adjusted HR 0.39, 95% CI 0.27-0.57). Corticosteroids increased superinfection (9.7% versus 2.7%) and deaths when controlled for indications (adjusted HR 1.73, 95% CI 1.14-2.62). Early NAI treatment was associated with shorter length of stay in a subanalysis. NAI treatment may improve survival of hospitalised influenza patients; benefit is greatest from, but not limited to, treatment started within 2 days of illness. Superinfections and corticosteroids increase mortality. Antiviral and non-antiviral management strategies should be considered.
Copyright ©ERS 2015.
Conflict of interest statement
Conflict of interest: Disclosures can be found alongside the online version of this article at
Figures
References
-
- World Health Organization (WHO). Influenza. www.who.int/influenza/en/ Date last accessed: September 1, 2014.
-
- Leo YS, Lye DC, Chow A. Influenza in the tropics. Lancet Infect Dis 2009; 9: 457–458. - PubMed
-
- Lee N, Ison MG. Diagnosis, management and outcomes of adults hospitalized with influenza. Antivir Ther 2012; 17: 143–157. - PubMed
-
- Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 2012; 12: 687–695. - PubMed
-
- Lee N, Chan PK, Lui GC, et al. Complications and outcomes of pandemic 2009 influenza A (H1N1) virus infection in hospitalized adults: how do they differ from those in seasonal influenza? J Infect Dis 2011; 203: 1739–1747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
