9-Phenanthrol inhibits recombinant and arterial myocyte TMEM16A channels
- PMID: 25573456
- PMCID: PMC4409899
- DOI: 10.1111/bph.13077
9-Phenanthrol inhibits recombinant and arterial myocyte TMEM16A channels
Abstract
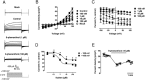
Background and purpose: In arterial smooth muscle cells (myocytes), intravascular pressure stimulates membrane depolarization and vasoconstriction (the myogenic response). Ion channels proposed to mediate pressure-induced depolarization include several transient receptor potential (TRP) channels, including TRPM4, and transmembrane protein 16A (TMEM16A), a Ca(2+) -activated Cl(-) channel (CaCC). 9-Phenanthrol, a putative selective TRPM4 channel inhibitor, abolishes myogenic tone in cerebral arteries, suggesting that either TRPM4 is essential for pressure-induced depolarization, upstream of activation of other ion channels or that 9-phenanthrol is non-selective. Here, we tested the hypothesis that 9-phenanthrol is also a TMEM16A channel blocker, an ion channel for which few inhibitors have been identified.
Experimental approach: Patch clamp electrophysiology was used to measure rat cerebral artery myocyte and human recombinant TMEM16A (rTMEM16A) currents or currents generated by recombinant bestrophin-1, another Ca(2+) -activated Cl(-) channel, expressed in HEK293 cells.
Key results: 9-Phenanthrol blocked myocyte TMEM16A currents activated by either intracellular Ca(2+) or Eact , a TMEM16A channel activator. In contrast, 9-phenanthrol did not alter recombinant bestrophin-1 currents. 9-Phenanthrol reduced arterial myocyte TMEM16A currents with an IC50 of ∼12 μM. Cell-attached patch recordings indicated that 9-phenanthrol reduced single rTMEM16A channel open probability and mean open time, and increased mean closed time without affecting the amplitude.
Conclusions and implications: These data identify 9-phenanthrol as a novel TMEM16A channel blocker and provide an explanation for the previous observation that 9-phenanthrol abolishes myogenic tone when both TRPM4 and TMEM16A channels contribute to this response. 9-Phenanthrol may be a promising candidate from which to develop TMEM16A channel-specific inhibitors.
© 2015 The British Pharmacological Society.
Figures




Similar articles
-
TMEM16A/ANO1 channels contribute to the myogenic response in cerebral arteries.Circ Res. 2012 Sep 28;111(8):1027-36. doi: 10.1161/CIRCRESAHA.112.277145. Epub 2012 Aug 7. Circ Res. 2012. PMID: 22872152 Free PMC article.
-
Pharmacological inhibition of TRPM4 hyperpolarizes vascular smooth muscle.Am J Physiol Cell Physiol. 2010 Nov;299(5):C1195-202. doi: 10.1152/ajpcell.00269.2010. Epub 2010 Sep 8. Am J Physiol Cell Physiol. 2010. PMID: 20826763 Free PMC article.
-
TMEM16A channels generate Ca²⁺-activated Cl⁻ currents in cerebral artery smooth muscle cells.Am J Physiol Heart Circ Physiol. 2011 Nov;301(5):H1819-27. doi: 10.1152/ajpheart.00404.2011. Epub 2011 Aug 19. Am J Physiol Heart Circ Physiol. 2011. PMID: 21856902 Free PMC article.
-
The TRPM4 channel inhibitor 9-phenanthrol.Br J Pharmacol. 2014 Apr;171(7):1600-13. doi: 10.1111/bph.12582. Br J Pharmacol. 2014. PMID: 24433510 Free PMC article. Review.
-
Bestrophin and TMEM16-Ca(2+) activated Cl(-) channels with different functions.Cell Calcium. 2009 Oct;46(4):233-41. doi: 10.1016/j.ceca.2009.09.003. Epub 2009 Sep 26. Cell Calcium. 2009. PMID: 19783045 Review.
Cited by
-
Thermosensitive TRPV4 channels mediate temperature-dependent microglia movement.Proc Natl Acad Sci U S A. 2021 Apr 27;118(17):e2012894118. doi: 10.1073/pnas.2012894118. Proc Natl Acad Sci U S A. 2021. PMID: 33888579 Free PMC article.
-
Agonism of the TMEM16A calcium-activated chloride channel modulates airway smooth muscle tone.Am J Physiol Lung Cell Mol Physiol. 2020 Feb 1;318(2):L287-L295. doi: 10.1152/ajplung.00552.2018. Epub 2019 Nov 20. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 31747299 Free PMC article.
-
Species-Specific Effects of Cation Channel TRPM4 Small-Molecule Inhibitors.Front Pharmacol. 2021 Jul 12;12:712354. doi: 10.3389/fphar.2021.712354. eCollection 2021. Front Pharmacol. 2021. PMID: 34335274 Free PMC article.
-
Defining the ionic mechanisms of optogenetic control of vascular tone by channelrhodopsin-2.Br J Pharmacol. 2018 Jun;175(11):2028-2045. doi: 10.1111/bph.14183. Epub 2018 Apr 17. Br J Pharmacol. 2018. PMID: 29486056 Free PMC article.
-
TRPM4 Inhibition: An Unexpected Mechanism of NO-Induced Vasodilatation.Function (Oxf). 2022 Mar 24;3(2):zqac007. doi: 10.1093/function/zqac007. eCollection 2022. Function (Oxf). 2022. PMID: 35359910 Free PMC article. No abstract available.
References
-
- Amarouch MY, Syam N, Abriel H. Biochemical, single-channel, whole-cell patch clamp, and pharmacological analyses of endogenous TRPM4 channels in HEK293 cells. Neurosci Lett. 2013;541:105–110. - PubMed
-
- Caputo A, Caci E, Ferrara L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride activity. Science. 2008;322:590–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

