Schizandrin ameliorates ovariectomy-induced memory impairment, potentiates neurotransmission and exhibits antioxidant properties
- PMID: 25573619
- PMCID: PMC4409901
- DOI: 10.1111/bph.13078
Schizandrin ameliorates ovariectomy-induced memory impairment, potentiates neurotransmission and exhibits antioxidant properties
Abstract
Background and purpose: Schizandrin (SCH) has been reported to prevent or reduce learning and memory defects. However, it is not known whether SCH ameliorates cognitive impairments induced by oestrogen deficiency. In the present study, we investigated the effect of SCH on memory in ovariectomized (OVX) and non-OVX rats.
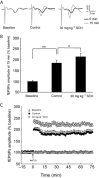
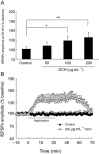
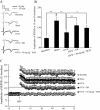
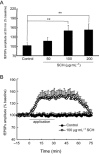
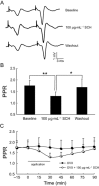
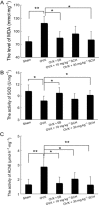
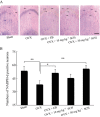
Experimental approach: A passive avoidance test was used to evaluate the effect of SCH on memory. Field EPSPs were recorded in hippocampal slices using an electrophysiological method. In OVX rats, biochemical parameters in the bilateral hippocampus were measured; these included superoxide dismutase (SOD), malondialdehyde (MDA) and AChE. Also, the number of NADPH-diaphorase (NADPH-d) positive neurons was counted by NADPH-d histochemistry staining technique.
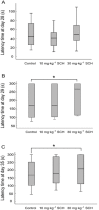
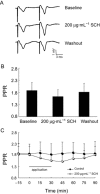
Key results: Oral SCH improved the memory and facilitated the induction of long-term potentiation in non-OVX and OVX rats; this effect was more obvious in OVX rats. Similarly, SCH perfusion enhanced synaptic transmission in hippocampal slices from both non-OVX and OVX rats. However, SCH perfusion reduced the ratio of paired-pulse facilitation only in OVX but not in non-OVX rats. In addition, SCH decreased AChE activity and MDA level and increased SOD activity and the number of NADPH-d-positive neurons in OVX rats.
Conclusions and implications: SCH improves memory in OVX rats and its potential mechanisms may include a reduction in the loss of hippocampal NADPH-d positive neurons, an increase of antioxidant properties and a potentiation of synaptic transmission that possibly involves to enhance cholinergic function. Overall, our findings indicate that SCH has potential as a therapeutic strategy for the cognitive dysfunctions associated with the menopause.
© 2015 The British Pharmacological Society.
Figures

Similar articles
-
Schizandrin, an antioxidant lignan from Schisandra chinensis, ameliorates Aβ1-42-induced memory impairment in mice.Oxid Med Cell Longev. 2012;2012:721721. doi: 10.1155/2012/721721. Epub 2012 Jul 4. Oxid Med Cell Longev. 2012. PMID: 22829961 Free PMC article.
-
Deoxyschizandrin isolated from the fruits of Schisandra chinensis ameliorates Aβ₁₋₄₂-induced memory impairment in mice.Planta Med. 2012 Aug;78(12):1332-6. doi: 10.1055/s-0032-1315019. Epub 2012 Jul 6. Planta Med. 2012. PMID: 22773410
-
[Effects of schizandrins on learning-memory disorder in mice].Zhongguo Zhong Yao Za Zhi. 2011 Dec;36(23):3310-4. Zhongguo Zhong Yao Za Zhi. 2011. PMID: 22393742 Chinese.
-
Schisandrin B: A Double-Edged Sword in Nonalcoholic Fatty Liver Disease.Oxid Med Cell Longev. 2016;2016:6171658. doi: 10.1155/2016/6171658. Epub 2016 Oct 26. Oxid Med Cell Longev. 2016. PMID: 27847552 Free PMC article. Review.
-
The anti-dementia drug candidate, (-)-clausenamide, improves memory impairment through its multi-target effect.Pharmacol Ther. 2016 Jun;162:179-87. doi: 10.1016/j.pharmthera.2016.01.002. Epub 2016 Jan 23. Pharmacol Ther. 2016. PMID: 26812265 Review.
Cited by
-
Can Dexmedetomidine Be Effective in the Protection of Radiotherapy-Induced Brain Damage in the Rat?Neurotox Res. 2021 Aug;39(4):1338-1351. doi: 10.1007/s12640-021-00379-1. Epub 2021 May 31. Neurotox Res. 2021. PMID: 34057703
-
Cordycepin Ameliorates Synaptic Dysfunction and Dendrite Morphology Damage of Hippocampal CA1 via A1R in Cerebral Ischemia.Front Cell Neurosci. 2021 Dec 24;15:783478. doi: 10.3389/fncel.2021.783478. eCollection 2021. Front Cell Neurosci. 2021. PMID: 35002628 Free PMC article.
-
In Silico Analysis, Anticonvulsant Activity, and Toxicity Evaluation of Schisandrin B in Zebrafish Larvae and Mice.Int J Mol Sci. 2023 Aug 18;24(16):12949. doi: 10.3390/ijms241612949. Int J Mol Sci. 2023. PMID: 37629132 Free PMC article.
-
A comprehensive review of Schisandra chinensis lignans: pharmacokinetics, pharmacological mechanisms, and future prospects in disease prevention and treatment.Chin Med. 2025 Apr 9;20(1):47. doi: 10.1186/s13020-025-01096-z. Chin Med. 2025. PMID: 40205412 Free PMC article. Review.
-
A comprehensive review on Schisandrin and its pharmacological features.Naunyn Schmiedebergs Arch Pharmacol. 2024 Feb;397(2):783-794. doi: 10.1007/s00210-023-02687-z. Epub 2023 Sep 1. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37658213 Review.
References
-
- Cai ZL, Wang CY, Gu XY, Wang NJ, Wang JJ, Liu WX, et al. Tenuigenin ameliorates learning and memory impairments induced by ovariectomy. Physiol Behav. 2013;118:112–117. - PubMed
-
- Chien WL, Liang KC, Teng CM, Kuo SC, Lee FY, Fu WM. Enhancement of learning behaviour by a potent nitric oxide-guanylate cyclase activator YC-1. Eur J Neurosci. 2005;21:1679–1688. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

