PPARγ deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage
- PMID: 25573665
- PMCID: PMC4345902
- DOI: 10.1136/annrheumdis-2014-205743
PPARγ deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage
Abstract
Objectives: We have previously shown that peroxisome proliferator-activated receptor gamma (PPARγ), a transcription factor, is essential for the normal growth and development of cartilage. In the present study, we created inducible cartilage-specific PPARγ knockout (KO) mice and subjected these mice to the destabilisation of medial meniscus (DMM) model of osteoarthritis (OA) to elucidate the specific in vivo role of PPARγ in OA pathophysiology. We further investigated the downstream PPARγ signalling pathway responsible for maintaining cartilage homeostasis.
Methods: Inducible cartilage-specific PPARγ KO mice were generated and subjected to DMM model of OA. We also created inducible cartilage-specific PPARγ/mammalian target for rapamycin (mTOR) double KO mice to dissect the PPARγ signalling pathway in OA.
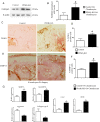
Results: Compared with control mice, PPARγ KO mice exhibit accelerated OA phenotype with increased cartilage degradation, chondrocyte apoptosis, and the overproduction of OA inflammatory/catabolic factors associated with the increased expression of mTOR and the suppression of key autophagy markers. In vitro rescue experiments using PPARγ expression vector reduced mTOR expression, increased expression of autophagy markers and reduced the expression of OA inflammatory/catabolic factors, thus reversing the phenotype of PPARγ KO mice chondrocytes. To dissect the in vivo role of mTOR pathway in PPARγ signalling, we created and subjected PPARγ-mTOR double KO mice to the OA model to see if the genetic deletion of mTOR in PPARγ KO mice (double KO) can rescue the accelerated OA phenotype observed in PPARγ KO mice. Indeed, PPARγ-mTOR double KO mice exhibit significant protection/reversal from OA phenotype.
Significance: PPARγ maintains articular cartilage homeostasis, in part, by regulating mTOR pathway.
Keywords: Arthritis; Chondrocytes; Osteoarthritis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures





Comment in
-
PPARγ/mTOR signalling: striking the right balance in cartilage homeostasis.Ann Rheum Dis. 2015 Mar;74(3):477-9. doi: 10.1136/annrheumdis-2014-206884. Epub 2015 Jan 14. Ann Rheum Dis. 2015. PMID: 25589512 Free PMC article. No abstract available.
Similar articles
-
Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis.Ann Rheum Dis. 2015 Jul;74(7):1432-40. doi: 10.1136/annrheumdis-2013-204599. Epub 2014 Mar 20. Ann Rheum Dis. 2015. PMID: 24651621
-
mTORC1 activation downregulates FGFR3 and PTH/PTHrP receptor in articular chondrocytes to initiate osteoarthritis.Osteoarthritis Cartilage. 2017 Jun;25(6):952-963. doi: 10.1016/j.joca.2016.12.024. Epub 2016 Dec 31. Osteoarthritis Cartilage. 2017. PMID: 28043938
-
Regulated in Development and DNA Damage Response 1 Deficiency Impairs Autophagy and Mitochondrial Biogenesis in Articular Cartilage and Increases the Severity of Experimental Osteoarthritis.Arthritis Rheumatol. 2017 Jul;69(7):1418-1428. doi: 10.1002/art.40104. Epub 2017 Jun 2. Arthritis Rheumatol. 2017. PMID: 28334504 Free PMC article.
-
Krüppel-like factor 15 deficiency exacerbates osteoarthritis through reduced expression of peroxisome proliferator-activated receptor gamma signaling in mice.Osteoarthritis Cartilage. 2024 Jan;32(1):28-40. doi: 10.1016/j.joca.2023.08.009. Epub 2023 Aug 28. Osteoarthritis Cartilage. 2024. PMID: 37648149
-
The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review.Osteoarthritis Cartilage. 2020 Apr;28(4):400-409. doi: 10.1016/j.joca.2020.02.027. Epub 2020 Feb 18. Osteoarthritis Cartilage. 2020. PMID: 32081707 Review.
Cited by
-
Salvianolic Acid A Has Anti-Osteoarthritis Effect In Vitro and In Vivo.Front Pharmacol. 2020 Jun 3;11:682. doi: 10.3389/fphar.2020.00682. eCollection 2020. Front Pharmacol. 2020. PMID: 32581777 Free PMC article.
-
Cellular ageing mechanisms in osteoarthritis.Mamm Genome. 2016 Aug;27(7-8):421-9. doi: 10.1007/s00335-016-9641-z. Epub 2016 May 23. Mamm Genome. 2016. PMID: 27215642 Free PMC article. Review.
-
Osteoarthritis: pathogenic signaling pathways and therapeutic targets.Signal Transduct Target Ther. 2023 Feb 3;8(1):56. doi: 10.1038/s41392-023-01330-w. Signal Transduct Target Ther. 2023. PMID: 36737426 Free PMC article. Review.
-
The role of AGEs in pathogenesis of cartilage destruction in osteoarthritis.Bone Joint Res. 2022 May;11(5):292-300. doi: 10.1302/2046-3758.115.BJR-2021-0334.R1. Bone Joint Res. 2022. PMID: 35549515 Free PMC article.
-
Low-Input RNA-Sequencing in Patients with Cartilage Lesions, Osteoarthritis, and Healthy Cartilage.Cartilage. 2021 Dec;13(1_suppl):550S-562S. doi: 10.1177/19476035211057245. Epub 2021 Nov 15. Cartilage. 2021. PMID: 34775802 Free PMC article.
References
-
- Blaney Davidson EN, Vitters EL, van der Kraan PM, et al. . Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis 2006;65:1414–21. 10.1136/ard.2005.045971 - DOI - PMC - PubMed
-
- Monemdjou R, Fahmi H, Kapoor M. Synovium in the pathophysiology of osteoarthritis. Therapy 2010;7:661–8. 10.2217/thy.10.72 - DOI
-
- Monemdjou R, Fahmi H, Pelletier J-P, et al. . Metalloproteases in the Pathogenesis of Osteoarthritis. Int J Adv Rheumatol 2010;8:103–10..
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

