Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2
- PMID: 25573752
- PMCID: PMC4396602
- DOI: 10.1096/fj.14-265066
Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2
Abstract
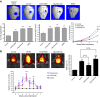
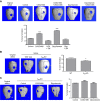
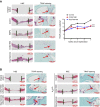
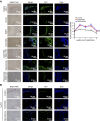
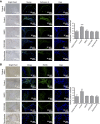
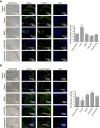
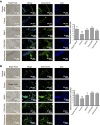
Promoting bone regeneration and repair of bone defects is a need that has not been well met to date. We have previously found that adenosine, acting via A2A receptors (A2AR) promotes wound healing and inhibits inflammatory osteolysis and hypothesized that A2AR might be a novel target to promote bone regeneration. Therefore, we determined whether direct A2AR stimulation or increasing endogenous adenosine concentrations via purine transport blockade with dipyridamole regulates bone formation. We determined whether coverage of a 3 mm trephine defect in a mouse skull with a collagen scaffold soaked in saline, bone morphogenetic protein-2 (BMP-2; 200 ng), 1 μM CGS21680 (A2AR agonist, EC50 = 160 nM), or 1 μM dipyridamole (EC50 = 32 nM) promoted bone regeneration. Microcomputed tomography examination demonstrated that CGS21680 and dipyridamole markedly enhanced bone regeneration as well as BMP-2 8 wk after surgery (60 ± 2%, 79 ± 2%, and 75 ± 1% bone regeneration, respectively, vs. 32 ± 2% in control, P < 0.001). Blockade by a selective A2AR antagonist (ZM241385, 1 μM) or deletion of A2AR abrogated the effect of CGS21680 and dipyridamole on bone regeneration. Both CGS21680 and dipyridamole treatment increased alkaline phosphatase-positive osteoblasts and diminished tartrate resistance acid phosphatase-positive osteoclasts in the defects. In vivo imaging with a fluorescent dye for new bone formation revealed a strong fluorescent signal in treated animals that was equivalent to BMP-2. In conclusion, stimulation of A2AR by specific agonists or by increasing endogenous adenosine levels stimulates new bone formation as well as BMP-2 and represents a novel approach to stimulating bone regeneration.
Keywords: CGS21680; bone formation; dipyridamole; osteoblast; osteoclast.
© FASEB.
Figures
References
-
- Schindeler A., McDonald M. M., Bokko P., Little D. G. (2008) Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 19, 459–466 - PubMed
-
- Tsiridis E., Upadhyay N., Giannoudis P. (2007) Molecular aspects of fracture healing: which are the important molecules? Injury 38(Suppl 1), S11–S25 - PubMed
-
- Pape H. C., Marcucio R., Humphrey C., Colnot C., Knobe M., Harvey E. J. (2010) Trauma-induced inflammation and fracture healing. J. Orthop. Trauma 24, 522–525 - PubMed
-
- Riley E. H., Lane J. M., Urist M. R., Lyons K. M., Lieberman J. R. (1996) Bone morphogenetic protein-2: biology and applications. Clin. Orthop. Relat. Res. (324):39–46 - PubMed
-
- Boden S. D., Kang J., Sandhu H., Heller J. G. (2002) Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine 27, 2662–2673 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016087/CA/NCI NIH HHS/United States
- UL1TR000038/TR/NCATS NIH HHS/United States
- HL100815/HL/NHLBI NIH HHS/United States
- R01 AR056672/AR/NIAMS NIH HHS/United States
- AR046121/AR/NIAMS NIH HHS/United States
- UL1 TR000038/TR/NCATS NIH HHS/United States
- UL1 TR001445/TR/NCATS NIH HHS/United States
- RC1 HL100815/HL/NHLBI NIH HHS/United States
- AR56672/AR/NIAMS NIH HHS/United States
- R01 AR054897/AR/NIAMS NIH HHS/United States
- P30 AR046121/AR/NIAMS NIH HHS/United States
- R56 AR056672/AR/NIAMS NIH HHS/United States
- 5-P30CA16087-310/CA/NCI NIH HHS/United States
- AR54897/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

