Aquaporin-1 retards renal cyst development in polycystic kidney disease by inhibition of Wnt signaling
- PMID: 25573755
- PMCID: PMC4396615
- DOI: 10.1096/fj.14-260828
Aquaporin-1 retards renal cyst development in polycystic kidney disease by inhibition of Wnt signaling
Abstract
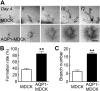
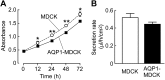
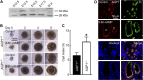
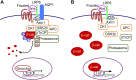
Water channel aquaporin-1 (AQP1) is expressed at epithelial cell plasma membranes in renal proximal tubules and thin descending limb of Henle. Recently, AQP1 was reported to interact with β-catenin. Here we investigated the relationship between AQP1 and Wnt signaling in in vitro and in vivo models of autosomal dominant polycystic kidney disease (PKD). AQP1 overexpression decreased β-catenin and cyclinD1 expression, suggesting down-regulation of Wnt signaling, and coimmunoprecipitation showed AQP1 interaction with β-catenin, glycogen synthase kinase 3β, LRP6, and Axin1. AQP1 inhibited cyst development and promoted branching in matrix-grown MDCK cells. In embryonic kidney cultures, AQP1 deletion increased cyst development by up to ∼ 40%. Kidney size and cyst number were significantly greater in AQP1-null PKD mice than in AQP1-expressing PKD mice, with the difference mainly attributed to a greater number of proximal tubule cysts. Biochemical analysis revealed decreased β-catenin phosphorylation and increased β-catenin expression in AQP1-null PKD mice, suggesting enhanced Wnt signaling. These results implicate AQP1 as a novel determinant in renal cyst development that may involve inhibition of Wnt signaling by an AQP1-macromolecular signaling complex.
Keywords: ADPKD; MDCK; destruction complex; water channel.
© FASEB.
Figures







Similar articles
-
Expression of aquaporins-1 and -2 during nephrogenesis and in autosomal dominant polycystic kidney disease.Am J Physiol. 1996 Jul;271(1 Pt 2):F169-83. doi: 10.1152/ajprenal.1996.271.1.F169. Am J Physiol. 1996. PMID: 8760258
-
TAZ/Wnt-β-catenin/c-MYC axis regulates cystogenesis in polycystic kidney disease.Proc Natl Acad Sci U S A. 2020 Nov 17;117(46):29001-29012. doi: 10.1073/pnas.2009334117. Epub 2020 Oct 29. Proc Natl Acad Sci U S A. 2020. PMID: 33122431 Free PMC article.
-
β-catenin ablation exacerbates polycystic kidney disease progression.Hum Mol Genet. 2019 Jan 15;28(2):230-244. doi: 10.1093/hmg/ddy309. Hum Mol Genet. 2019. PMID: 30265301
-
Polycystic kidney disease: the complexity of planar cell polarity and signaling during tissue regeneration and cyst formation.Biochim Biophys Acta. 2011 Oct;1812(10):1249-55. doi: 10.1016/j.bbadis.2011.05.005. Epub 2011 May 27. Biochim Biophys Acta. 2011. PMID: 21640821 Review.
-
Physiological and pathological impact of AQP1 knockout in mice.Biosci Rep. 2019 May 14;39(5):BSR20182303. doi: 10.1042/BSR20182303. Print 2019 May 31. Biosci Rep. 2019. PMID: 31023968 Free PMC article. Review.
Cited by
-
Overexpression of Aquaporin 1 in Synovium Aggravates Rat Collagen-Induced Arthritis Through Regulating β-Catenin Signaling: An in vivo and in vitro Study.J Inflamm Res. 2020 Oct 8;13:701-712. doi: 10.2147/JIR.S271664. eCollection 2020. J Inflamm Res. 2020. PMID: 33116749 Free PMC article.
-
Aquaporins in Urinary System.Adv Exp Med Biol. 2023;1398:155-177. doi: 10.1007/978-981-19-7415-1_11. Adv Exp Med Biol. 2023. PMID: 36717493
-
Aquaporins in Renal Diseases.Int J Mol Sci. 2019 Jan 16;20(2):366. doi: 10.3390/ijms20020366. Int J Mol Sci. 2019. PMID: 30654539 Free PMC article. Review.
-
Inhibitory effect of AQP1 silencing on adhesion and angiogenesis in ectopic endometrial cells of mice with endometriosis through activating the Wnt signaling pathway.Cell Cycle. 2019 Sep;18(17):2026-2039. doi: 10.1080/15384101.2019.1637202. Epub 2019 Jul 15. Cell Cycle. 2019. PMID: 31251110 Free PMC article.
-
Differential Roles of Cysteinyl Cathepsins in TGF-β Signaling and Tissue Fibrosis.iScience. 2019 Sep 27;19:607-622. doi: 10.1016/j.isci.2019.08.014. Epub 2019 Aug 9. iScience. 2019. PMID: 31446224 Free PMC article.
References
-
- Ma T., Yang B., Gillespie A., Carlson E. J., Epstein C. J., Verkman A. S. (1998) Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J. Biol. Chem. 273, 4296–4299 - PubMed
-
- Verkman A. S. (2011) Aquaporins at a glance. J. Cell Sci. 124, 2107–2112 - PubMed
-
- Noda Y., Sohara E., Ohta E., Sasaki S. (2010) Aquaporins in kidney pathophysiology. Nat. Rev. Nephrol. 6, 168–178 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

