Chemobrain: a critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy
- PMID: 25573802
- PMCID: PMC4750385
- DOI: 10.1016/j.cyto.2014.12.006
Chemobrain: a critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy
Abstract
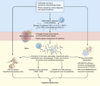
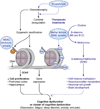
One consequence of modern cancer therapy is chemotherapy related cognitive dysfunction or "chemobrain", the subjective experience of cognitive deficits at any point during or following chemotherapy. Chemobrain, a well-established clinical syndrome, has become an increasing concern because the number of long-term cancer survivors is growing dramatically. There is strong evidence that correlates changes in peripheral cytokines with the development of chemobrain in commonly used chemotherapeutic drugs for different types of cancer. However, the mechanisms by which these cytokines elicit change in the central nervous system are still unclear. In this review, we hypothesize that the administration of chemotherapy agents initiates a cascade of biological changes, with short-lived alterations in the cytokine milieu inducing persistent epigenetic alterations. These epigenetic changes lead to changes in gene expression, alterations in metabolic activity and neuronal transmission that are responsible for generating the subjective experience of cognition. This speculative but testable hypothesis should help to gain a comprehensive understanding of the mechanism underlying cognitive dysfunction in cancer patients. Such knowledge is critical to identify pharmaceutical targets with the potential to prevent and treat cancer-treatment related cognitive dysfunction and similar disorders.
Keywords: Cancer chemotherapy; Chemobrain; Cognition dysfunction; Cytokines and epigenetics.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
References
-
- Berger A, Shuster JL, Von Roenn JH. Principles and practice of palliative care and supportive oncology. 4th. xvii. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013.
-
- Wefel JS, et al. ‘Chemobrain’ in breast carcinoma?: A prologue. Cancer. 2004;101(3):466–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

