Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib
- PMID: 25573991
- PMCID: PMC4467871
- DOI: 10.1182/blood-2014-09-603670
Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib
Abstract
Ibrutinib is a Bruton tyrosine kinase inhibitor approved for the treatment of patients with relapsed refractory chronic lymphocytic leukemia (RR-CLL). We describe the characteristics, causes of discontinuation, and outcomes in patients who discontinued treatment with ibrutinib. One hundred twenty-seven patients were enrolled in various clinical trials of ibrutinib, with or without rituximab, at our center. Thirty-three (26%) patients have discontinued ibrutinib to date. The majority of those patients had high-risk features: 94% with unmutated immunoglobulin heavy chain variable gene rearrangement, 58% with del(17p) by fluorescence in situ hybridization, and 54% with a complex karyotype. Causes of discontinuation were disease transformation (7), progressive CLL (7), stem cell transplantation (3), adverse events (11), serious adverse events/deaths (3), and miscellaneous reasons (2). Twenty five patients (76%) died after discontinuing ibrutinib; the median overall survival was 3.1 months after discontinuation. Most patients with RR-CLL who discontinued ibrutinib early were difficult to treat and had poor outcomes.
© 2015 by The American Society of Hematology.
Figures
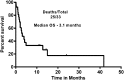
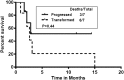
Comment in
-
Life after ibrutinib? A new unmet need in CLL.Blood. 2015 Mar 26;125(13):2013-4. doi: 10.1182/blood-2015-02-622472. Blood. 2015. PMID: 25814485
References
-
- Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014;14(4):219–232. - PubMed
-
- O'Brien S, Furman RR, Fowler N, et al. The Bruton’s Tyrosine Kinase (BTK) Inhibitor Ibrutinib (PCI-32765) Monotherapy Demonstrates Long-Term Safety and Durability Of Response In Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL) Patients In An Open-Label Extension Study. Blood. 2013;122(21):4163. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

