Biosynthetically Distinct Cytotoxic Polyketides from Setophoma terrestris
- PMID: 25574154
- PMCID: PMC4283843
- DOI: 10.1002/ejoc.201402984
Biosynthetically Distinct Cytotoxic Polyketides from Setophoma terrestris
Abstract
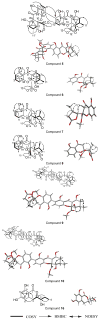
Sixteen polyketides belonging to diverse structural classes, including monomeric/dimeric tetrahydroxanthones and resorcylic acid lactones, were isolated from an organic extract of a fungal culture Setophoma terrestris (MSX45109) using bioactivity-directed fractionation as part of a search for anticancer leads from filamentous fungi. Of these, six were new: penicillixanthone B (5), blennolide H (6), 11-deoxy blennolide D (7), blennolide I (9), blennolide J (10), and pyrenomycin (16). The known compounds were: secalonic acid A (1), secalonic acid E (2), secalonic acid G (3), penicillixanthone A (4), paecilin B (8), aigialomycin A (11), hypothemycin (12), dihydrohypothemycin (13), pyrenochaetic acid C (14), and nidulalin B (15). The structures were elucidated using a set of spectroscopic and spectrometric techniques; the absolute configurations of compounds 1-10 were determined using ECD spectroscopy combined with time-dependent density functional theory (TDDFT) calculations, while a modified Mosher's ester method was used for compound 16. The cytotoxic activities of compounds (1-15) were evaluated using the MDA-MB-435 (melanoma) and SW-620 (colon) cancer cell lines. Compounds 1, 4, and 12 were the most potent with IC50 values ranging from 0.16 to 2.14 μM. When tested against a panel of bacteria and fungi, compounds 3 and 5 showed promising activity against the Gram-positive bacterium Micrococcus luteus with MIC values of 5 and 15 μg/mL, respectively.
Keywords: Cytotoxicity; ergochromes; fungus; resorcylic acid lactones; secalonic acids.
Figures




Similar articles
-
Aculeaxanthones A-E, new xanthones from the marine-derived fungus Aspergillus aculeatinus WHUF0198.Front Microbiol. 2023 Feb 27;14:1138830. doi: 10.3389/fmicb.2023.1138830. eCollection 2023. Front Microbiol. 2023. PMID: 36922969 Free PMC article.
-
New mono- and dimeric members of the secalonic acid family: blennolides A-G isolated from the fungus Blennoria sp.Chemistry. 2008;14(16):4913-23. doi: 10.1002/chem.200800035. Chemistry. 2008. PMID: 18425741
-
Greensporones: resorcylic acid lactones from an aquatic Halenospora sp.J Nat Prod. 2014 Sep 26;77(9):2088-98. doi: 10.1021/np500497r. Epub 2014 Aug 5. J Nat Prod. 2014. PMID: 25093280 Free PMC article.
-
Natural resorcylic acid lactones: A chemical biology approach for anticancer activity.Drug Discov Today. 2022 Feb;27(2):547-557. doi: 10.1016/j.drudis.2021.10.001. Epub 2021 Oct 13. Drug Discov Today. 2022. PMID: 34655796 Review.
-
Isolation, Structure Determination, and Semisynthesis of Diphenazine Compounds from a Deep-Sea-Derived Strain of the Fungus Cystobasidium laryngis and Their Biological Activities.J Nat Prod. 2022 Apr 22;85(4):857-865. doi: 10.1021/acs.jnatprod.1c00985. Epub 2022 Mar 18. J Nat Prod. 2022. PMID: 35302779 Review.
Cited by
-
Engineering Fluorine into Verticillins (Epipolythiodioxopiperazine Alkaloids) via Precursor-Directed Biosynthesis.J Nat Prod. 2019 Nov 22;82(11):3104-3110. doi: 10.1021/acs.jnatprod.9b00711. Epub 2019 Oct 21. J Nat Prod. 2019. PMID: 31633350 Free PMC article.
-
Semisynthetic Derivatives of the Verticillin Class of Natural Products through Acylation of the C11 Hydroxy Group.ACS Med Chem Lett. 2021 Mar 19;12(4):625-630. doi: 10.1021/acsmedchemlett.1c00024. eCollection 2021 Apr 8. ACS Med Chem Lett. 2021. PMID: 33859802 Free PMC article.
-
A New Dihydrochromone Dimer and Other Secondary Metabolites from Cultures of the Marine Sponge-Associated Fungi Neosartorya fennelliae KUFA 0811 and Neosartorya tsunodae KUFC 9213.Mar Drugs. 2017 Dec 1;15(12):375. doi: 10.3390/md15120375. Mar Drugs. 2017. PMID: 29194412 Free PMC article.
-
Cytotoxic Homoisoflavones from the Bulbs of Bellevalia eigii.J Nat Prod. 2015 Jul 24;78(7):1708-15. doi: 10.1021/acs.jnatprod.5b00357. Epub 2015 Jul 6. J Nat Prod. 2015. PMID: 26147490 Free PMC article.
-
Phaeosphaeridiols A-C: Three New Compounds from Undescribed Phaeosphaeriaceae sp. SGSF723.J Fungi (Basel). 2022 Nov 11;8(11):1190. doi: 10.3390/jof8111190. J Fungi (Basel). 2022. PMID: 36422011 Free PMC article.
References
-
- El-Elimat T, Figueroa M, Ehrmann BM, Cech NB, Pearce CJ, Oberlies NH. J Nat Prod. 2013;76:1709–1716. - PMC - PubMed
- El-Elimat T, Figueroa M, Raja HA, Graf TN, Adcock AF, Kroll DJ, Day CS, Wani MC, Pearce CJ, Oberlies NH. J Nat Prod. 2013;76:382–387. - PMC - PubMed
- El-Elimat T, Zhang X, Jarjoura D, Moy FJ, Orjala J, Kinghorn AD, Pearce CJ, Oberlies NH. ACS Med Chem Lett. 2012;3:645–649. - PMC - PubMed
- Figueroa M, Graf TN, Ayers S, Adcock AF, Kroll DJ, Yang J, Swanson SM, Munoz-Acuna U, Carcache de Blanco EJ, Agrawal R, Wani MC, Darveaux BA, Pearce CJ, Oberlies NH. J Antibiot. 2012;65:559–564. - PMC - PubMed
- Sy-Cordero AA, Graf TN, Adcock AF, Kroll DJ, Shen Q, Swanson SM, Wani MC, Pearce CJ, Oberlies NH. J Nat Prod. 2011;74:2137–2142. - PMC - PubMed
- Sy-Cordero AA, Pearce CJ, Oberlies NH. J Antibiot. 2012;65:541–549. - PMC - PubMed
- Ayers S, Ehrmann BM, Adcock AF, Kroll DJ, Wani MC, Pearce CJ, Oberlies NH. Tetrahedron Lett. 2011;52:5733–5735. - PMC - PubMed
- Ayers S, Graf TN, Adcock AF, Kroll DJ, Matthew S, Carcache de Blanco EJ, Shen Q, Swanson SM, Wani MC, Pearce CJ, Oberlies NH. J Nat Prod. 2011;74:1126–1131. - PMC - PubMed
- Ayers S, Graf TN, Adcock AF, Kroll DJ, Shen Q, Swanson SM, Matthew S, Carcache de Blanco EJ, Wani MC, Darveaux BA, Pearce CJ, Oberlies NH. J Antibiot. 2012;65:3–8. - PMC - PubMed
- Ayers S, Graf TN, Adcock AF, Kroll DJ, Shen Q, Swanson SM, Wani MC, Darveaux BA, Pearce CJ, Oberlies NH. Tetrahedron Lett. 2011;52:5128–5230. - PMC - PubMed
-
- Braäse S, Encinas A, Keck J, Nising CF. Chem Rev. 2009;109:3903–3990. - PubMed
- Masters KS, Bräse S. Chem Rev. 2012;112:3717–3776. - PubMed
- Stoll A, Renz J, Brack A. Helv Chim Acta. 1952;35:2022–2034.
- Kurobane I, Iwahashi S, Fukuda A. Drugs Exp Clin Res. 1987;13:339–344. - PubMed
- Liao G, Zhou J, Wang H, Mao Z, Xiao W, Wang H, She Z, Zhu Y. Oncol Rep. 2010;23:387–395. - PubMed
- McPhee F, Caldera PS, Bemis GW, McDonagh AF, Kuntz ID, Craik CS. Biochem J. 1996;320(Pt 2):681–686. - PMC - PubMed
-
- Ito T, Masubuchi M. J Antibiot. 2014;67:353–360. - PubMed
- Yang JY, Sanchez LM, Rath CM, Liu X, Boudreau PD, Bruns N, Glukhov E, Wodtke A, de Felicio R, Fenner A, Wong WR, Linington RG, Zhang L, Debonsi HM, Gerwick WH, Dorrestein PC. J Nat Prod. 2013;76:1686–1699. - PMC - PubMed
- Klitgaard A, Iversen A, Andersen MR, Larsen TO, Frisvad JC, Nielsen KF. Anal Bioanal Chem. 2014;406:1933–1943. - PMC - PubMed
-
- Zhang W, Krohn K, Ullah Z, Florke U, Pescitelli G, Di Bari L, Antus S, Kurtan T, Rheinheimer J, Draeger S, Schulz B. Chem - Eur J. 2008;14:4913–4923. - PubMed
-
- Bräse S, Gläser F, Kramer CS. In: The Chemistry of Mycotoxins. Bräse S, Gläser F, Kramer C, Lindner S, Linsenmeier AM, Masters KS, Meister AC, Ruff BM, Zhong S, editors. Springer; Vienna: 2013. pp. 91–108. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
