Tumor necrosis factor-α suppresses the protein fractional synthesis rate of the small intestine stimulated by glutamine in rats
- PMID: 25574232
- PMCID: PMC4280961
- DOI: 10.3892/etm.2014.2129
Tumor necrosis factor-α suppresses the protein fractional synthesis rate of the small intestine stimulated by glutamine in rats
Abstract
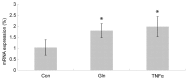
The objective of this study was to examine whether and how TNF-α affects glutamine-enhanced protein synthesis and the expression of the amino acid transporter ASCT2 in the small intestine at the mRNA and protein levels. A total of 30 male Sprague-Dawley rats were randomly assigned into three groups, namely the total parenteral nutrition (TPN; control), glutamine-treated (Gln), and glutamine- and tumor necrosis factor-α (TNF-α)-treated (TNF-α) groups. At 30 min prior to examination, all rats were mainlined with [L-15N]leucine. The concentration of TNF-α in plasma and of glutamine in plasma and the small intestine was measured. The fractional synthesis rate (FSR) of protein and the mRNA and protein expression levels of ASCT2 in the small intestine were assessed. The level of TNF-α was highest in the TNF-α group and the glutamine concentration was elevated to a greater extent in the TNF-α group than in the other two groups. However, the FSR of protein in the small intestine was significantly higher in the Gln group compared with that in the TNF-α group. The mRNA and protein expression levels of ASCT2 in the experimental groups were significantly higher that those in the control group, but did not differ significantly between the Gln and TNF-α groups. These results indicate that TNF-α may attenuate glutamine-stimulated protein synthesis in the small intestine in the early stage of sepsis in rats. The mechanism may be that TNF-α inhibits the function of the glutamine transporter in the uptake the glutamine into target cells for protein synthesis. This inhibition may occur at or following protein translation.
Keywords: ASCT2; fractional synthesis rate; glutamine; glutamine transporter; tumor necrosis factor-α.
Figures


References
-
- Bello G, Di Muzio F, Maviglia R, Antonelli M. New membranes for extracorporeal blood purification in septic conditions. Minerva Anestesiol. 2012;78:1265–1281. - PubMed
-
- Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr. 2001;131(9 Suppl):2515S–2522S. - PubMed
-
- Boelens PG, Nijveldt RJ, Houdijk AP, Meijer S, van Leeuwen PA. Glutamine alimentation in catabolic state. J Nutr. 2001;131:2569S–2577S. discussion 2590S. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
