Enhanced quantitative phase imaging in self-interference digital holographic microscopy using an electrically focus tunable lens
- PMID: 25574433
- PMCID: PMC4285600
- DOI: 10.1364/BOE.5.004213
Enhanced quantitative phase imaging in self-interference digital holographic microscopy using an electrically focus tunable lens
Abstract
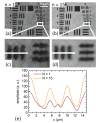
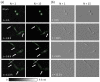
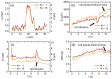
Self-interference digital holographic microscopy (DHM) has been found particular suitable for simplified quantitative phase imaging of living cells. However, a main drawback of the self-interference DHM principle are scattering patterns that are induced by the coherent nature of the laser light which affect the resolution for detection of optical path length changes. We present a simple and efficient technique for the reduction of coherent disturbances in quantitative phase images. Therefore, amplitude and phase of the sample illumination are modulated by an electrically focus tunable lens. The proposed method is in particular convenient with the self-interference DHM concept. Results from the characterization of the method show that a reduction of coherence induced disturbances up to 70 percent can be achieved. Finally, the performance for enhanced quantitative imaging of living cells is demonstrated.
Keywords: (090.1995) Digital holography; (110.0180) Microscopy; (110.1650) Coherence imaging; (120.5050) Phase measurement; (170.1530) Cell analysis.
Figures


Similar articles
-
A practical criterion for focusing of unstained cell samples using a digital holographic microscope.J Microsc. 2020 Aug;279(2):114-122. doi: 10.1111/jmi.12924. Epub 2020 Jun 5. J Microsc. 2020. PMID: 32441768
-
Coherent noise reduction in digital holographic microscopy by averaging multiple holograms recorded with a multimode laser.Opt Express. 2017 Sep 4;25(18):21815-21825. doi: 10.1364/OE.25.021815. Opt Express. 2017. PMID: 29041474
-
Advantages of Fresnel biprism-based digital holographic microscopy in quantitative phase imaging.J Biomed Opt. 2020 Aug;25(8):1-11. doi: 10.1117/1.JBO.25.8.086501. J Biomed Opt. 2020. PMID: 32755077 Free PMC article.
-
Sample and substrate preparation for exploring living neurons in culture with quantitative-phase imaging.Methods. 2018 Mar 1;136:90-107. doi: 10.1016/j.ymeth.2018.02.001. Epub 2018 Feb 10. Methods. 2018. PMID: 29438830 Review.
-
Review of quantitative phase-digital holographic microscopy: promising novel imaging technique to resolve neuronal network activity and identify cellular biomarkers of psychiatric disorders.Neurophotonics. 2014 Oct;1(2):020901. doi: 10.1117/1.NPh.1.2.020901. Epub 2014 Sep 22. Neurophotonics. 2014. PMID: 26157976 Free PMC article. Review.
Cited by
-
Developing a Reliable Holographic Flow Cyto-Tomography Apparatus by Optimizing the Experimental Layout and Computational Processing.Cells. 2022 Aug 19;11(16):2591. doi: 10.3390/cells11162591. Cells. 2022. PMID: 36010667 Free PMC article.
-
Unexpected localization of AQP3 and AQP4 induced by migration of primary cultured IMCD cells.Sci Rep. 2021 Jun 7;11(1):11930. doi: 10.1038/s41598-021-91369-y. Sci Rep. 2021. PMID: 34099798 Free PMC article.
-
Quantitative phase microscopies: accuracy comparison.Light Sci Appl. 2024 Oct 11;13(1):288. doi: 10.1038/s41377-024-01619-7. Light Sci Appl. 2024. PMID: 39394163 Free PMC article. Review.
-
Polychromatic digital holographic microscopy: a quasicoherent-noise-free imaging technique to explore the connectivity of living neuronal networks.Neurophotonics. 2020 Oct;7(4):040501. doi: 10.1117/1.NPh.7.4.040501. Epub 2020 Oct 16. Neurophotonics. 2020. PMID: 33094123 Free PMC article.
-
Real-time halo correction in phase contrast imaging.Biomed Opt Express. 2018 Jan 16;9(2):623-635. doi: 10.1364/BOE.9.000623. eCollection 2018 Feb 1. Biomed Opt Express. 2018. PMID: 29552399 Free PMC article.
References
-
- Popescu G., Quantitative phase imaging of cells and tissues (McGraw Hill, 2011).
-
- Shaked N., Zalevsky Z., Satterwhite L. L., eds., Biomedical Optical Phase Microscopy and Nanoscopy (Elsevier, 2012).
-
- Edwards C., Zhou R., Hwang S. W., McKeown S. J., Wang K., Bhaduri B., Ganti R., Yunker P. J., Yodh A. G., Rogers J. A., Goddard L. L., Popescu G., “Diffraction phase microscopy: monitoring nanoscale dynamics in materials science,” Appl. Opt. 53(27), G33–G43 (2014).10.1364/AO.53.000G33 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
