On the antimicrobial activity of various peptide-based dendrimers of similar architecture
- PMID: 25574818
- PMCID: PMC6272501
- DOI: 10.3390/molecules20010738
On the antimicrobial activity of various peptide-based dendrimers of similar architecture
Abstract
Antimicrobial drug resistance is a major human health threat. Among the many attempts to tackle this problem, the synthesis of antimicrobial compounds that mimic natural antimicrobial peptides appears as a promising approach. Peptide-based dendrimers can be designed to have higher potency than natural antimicrobial peptides and at the same time they can evade the bacterial defense system. Novel dendrimers with similar chemical structure but varying potency in terms of minimum inhibitory concentration were designed. The dependency between dendrimer structure and antibacterial activity as well as their capacity to attack model cell membranes was studied. The data suggests that supramolecular structure in terms of charge distribution and amphiphilicity, rather than net charge, is the main driver for disruption of cellular membranes and this correlates well with dendrimer hemolytic activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

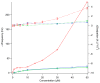


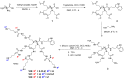
Similar articles
-
Optimizing Antimicrobial Peptide Dendrimers in Chemical Space.Angew Chem Int Ed Engl. 2018 Jul 9;57(28):8483-8487. doi: 10.1002/anie.201802837. Epub 2018 Jun 15. Angew Chem Int Ed Engl. 2018. PMID: 29767453
-
Synthesis, iron binding and antimicrobial properties of hexadentate 3-hydroxypyridinones-terminated dendrimers.Bioorg Med Chem Lett. 2018 Aug 1;28(14):2504-2512. doi: 10.1016/j.bmcl.2018.05.058. Epub 2018 May 30. Bioorg Med Chem Lett. 2018. PMID: 29886020
-
Nano-Sized Lipidated Dendrimers as Potent and Broad-Spectrum Antibacterial Agents.Macromol Rapid Commun. 2018 Dec;39(24):e1800622. doi: 10.1002/marc.201800622. Epub 2018 Nov 8. Macromol Rapid Commun. 2018. PMID: 30408252
-
Antimicrobial random peptide cocktails: a new approach to fight pathogenic bacteria.Chem Commun (Camb). 2019 Feb 12;55(14):2007-2014. doi: 10.1039/c8cc09961h. Chem Commun (Camb). 2019. PMID: 30688322 Review.
-
The development of antimicrobial γ-AApeptides.Future Med Chem. 2016 Jun;8(10):1101-10. doi: 10.4155/fmc-2016-0034. Epub 2016 Jun 10. Future Med Chem. 2016. PMID: 27284624 Free PMC article. Review.
Cited by
-
In Vitro Activity of the Novel Antimicrobial Peptide Dendrimer G3KL against Multidrug-Resistant Acinetobacter baumannii and Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2015 Dec;59(12):7915-8. doi: 10.1128/AAC.01853-15. Epub 2015 Oct 12. Antimicrob Agents Chemother. 2015. PMID: 26459893 Free PMC article.
-
Peptide Dendrimer-Based Antibacterial Agents: Synthesis and Applications.ACS Infect Dis. 2024 Apr 12;10(4):1034-1055. doi: 10.1021/acsinfecdis.3c00624. Epub 2024 Mar 1. ACS Infect Dis. 2024. PMID: 38428037 Free PMC article. Review.
-
Cationic Amphiphiles with Specificity against Gram-Positive and Gram-Negative Bacteria: Chemical Composition and Architecture Combat Bacterial Membranes.Langmuir. 2019 Apr 23;35(16):5557-5567. doi: 10.1021/acs.langmuir.9b00110. Epub 2019 Apr 10. Langmuir. 2019. PMID: 30888181 Free PMC article.
-
The Quest for Anti-inflammatory and Anti-infective Biomaterials in Clinical Translation.Front Bioeng Biotechnol. 2016 Sep 9;4:71. doi: 10.3389/fbioe.2016.00071. eCollection 2016. Front Bioeng Biotechnol. 2016. PMID: 27668213 Free PMC article. Review.
-
The Antimicrobial Peptide lin-SB056-1 and Its Dendrimeric Derivative Prevent Pseudomonas aeruginosa Biofilm Formation in Physiologically Relevant Models of Chronic Infections.Front Microbiol. 2019 Feb 8;10:198. doi: 10.3389/fmicb.2019.00198. eCollection 2019. Front Microbiol. 2019. PMID: 30800115 Free PMC article.
References
-
- Stromstedt A.A., Ringstad L., Schmidtchen A., Malmsten M. Interaction between amphiphilic peptides and phospholipid membranes. Curr. Opin. Colloid Interface Sci. 2010;15:467–478. doi: 10.1016/j.cocis.2010.05.006. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

