Genome-wide comparative analysis of atopic dermatitis and psoriasis gives insight into opposing genetic mechanisms
- PMID: 25574825
- PMCID: PMC4289690
- DOI: 10.1016/j.ajhg.2014.12.004
Genome-wide comparative analysis of atopic dermatitis and psoriasis gives insight into opposing genetic mechanisms
Erratum in
- Am J Hum Genet. 2015 Dec 3;97(6):933
Abstract
Atopic dermatitis and psoriasis are the two most common immune-mediated inflammatory disorders affecting the skin. Genome-wide studies demonstrate a high degree of genetic overlap, but these diseases have mutually exclusive clinical phenotypes and opposing immune mechanisms. Despite their prevalence, atopic dermatitis and psoriasis very rarely co-occur within one individual. By utilizing genome-wide association study and ImmunoChip data from >19,000 individuals and methodologies developed from meta-analysis, we have identified opposing risk alleles at shared loci as well as independent disease-specific loci within the epidermal differentiation complex (chromosome 1q21.3), the Th2 locus control region (chromosome 5q31.1), and the major histocompatibility complex (chromosome 6p21-22). We further identified previously unreported pleiotropic alleles with opposing effects on atopic dermatitis and psoriasis risk in PRKRA and ANXA6/TNIP1. In contrast, there was no evidence for shared loci with effects operating in the same direction on both diseases. Our results show that atopic dermatitis and psoriasis have distinct genetic mechanisms with opposing effects in shared pathways influencing epidermal differentiation and immune response. The statistical analysis methods developed in the conduct of this study have produced additional insight from previously published data sets. The approach is likely to be applicable to the investigation of the genetic basis of other complex traits with overlapping and distinct clinical features.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
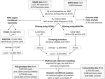




References
-
- Deckert S., Kopkow C., Schmitt J. Nonallergic comorbidities of atopic eczema: an overview of systematic reviews. Allergy. 2014;69:37–45. - PubMed
-
- Griffiths C.E., Barker J.N. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. - PubMed
-
- Parisi R., Symmons D.P., Griffiths C.E., Ashcroft D.M., Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J. Invest. Dermatol. 2013;133:377–385. - PubMed
-
- Henseler T., Christophers E. Disease concomitance in psoriasis. J. Am. Acad. Dermatol. 1995;32:982–986. - PubMed
Publication types
MeSH terms
Grants and funding
- 098439/WT_/Wellcome Trust/United Kingdom
- R01 AR042742/AR/NIAMS NIH HHS/United States
- R01AR062382/AR/NIAMS NIH HHS/United States
- 076113/WT_/Wellcome Trust/United Kingdom
- 085475/WT_/Wellcome Trust/United Kingdom
- 087436/Z/08/Z/WT_/Wellcome Trust/United Kingdom
- 087436/WT_/Wellcome Trust/United Kingdom
- 102858/Z/13/Z/WT_/Wellcome Trust/United Kingdom
- R01AR050511/AR/NIAMS NIH HHS/United States
- WT086398MA/WT_/Wellcome Trust/United Kingdom
- R01AR054966/AR/NIAMS NIH HHS/United States
- R01 AR054966/AR/NIAMS NIH HHS/United States
- R01 AR050511/AR/NIAMS NIH HHS/United States
- 092530/Z/10/Z/WT_/Wellcome Trust/United Kingdom
- G0802780/MRC_/Medical Research Council/United Kingdom
- G0700314/MRC_/Medical Research Council/United Kingdom
- R01 AR062886/AR/NIAMS NIH HHS/United States
- R01 AR065183/AR/NIAMS NIH HHS/United States
- R01 AR063611/AR/NIAMS NIH HHS/United States
- R01AR062886-01/AR/NIAMS NIH HHS/United States
- 098439/Z/12/Z/WT_/Wellcome Trust/United Kingdom
- R01AR042742/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

