Mutations in PNPLA6 are linked to photoreceptor degeneration and various forms of childhood blindness
- PMID: 25574898
- PMCID: PMC4356490
- DOI: 10.1038/ncomms6614
Mutations in PNPLA6 are linked to photoreceptor degeneration and various forms of childhood blindness
Abstract
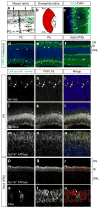
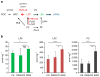
Blindness due to retinal degeneration affects millions of people worldwide, but many disease-causing mutations remain unknown. PNPLA6 encodes the patatin-like phospholipase domain containing protein 6, also known as neuropathy target esterase (NTE), which is the target of toxic organophosphates that induce human paralysis due to severe axonopathy of large neurons. Mutations in PNPLA6 also cause human spastic paraplegia characterized by motor neuron degeneration. Here we identify PNPLA6 mutations in childhood blindness in seven families with retinal degeneration, including Leber congenital amaurosis and Oliver McFarlane syndrome. PNPLA6 localizes mostly at the inner segment plasma membrane in photoreceptors and mutations in Drosophila PNPLA6 lead to photoreceptor cell death. We also report that lysophosphatidylcholine and lysophosphatidic acid levels are elevated in mutant Drosophila. These findings show a role for PNPLA6 in photoreceptor survival and identify phospholipid metabolism as a potential therapeutic target for some forms of blindness.
Conflict of interest statement
Figures




References
-
- Dudek BR, Richardson RJ. Evidence for the existence of neurotoxic esterase in neural and lymphatic tissue of the adult hen. Biochem Pharmacol. 1982;31:1117–1121. - PubMed
-
- Johnson MK. Initiation of organophosphate-induced delayed neuropathy. Neurobehav Toxicol Teratol. 1982;4:759–765. - PubMed
-
- Glynn P. Neuropathy target esterase and phospholipid deacylation. Biochim Biophys Acta. 2005;1736:87–93. - PubMed
-
- Glynn P. NTE: one target protein for different toxic syndromes with distinct mechanisms? Bioessays. 2003;25:742–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

