Persistence of frequently transmitted drug-resistant HIV-1 variants can be explained by high viral replication capacity
- PMID: 25575025
- PMCID: PMC4263067
- DOI: 10.1186/s12977-014-0105-9
Persistence of frequently transmitted drug-resistant HIV-1 variants can be explained by high viral replication capacity
Abstract
Background: In approximately 10% of newly diagnosed individuals in Europe, HIV-1 variants harboring transmitted drug resistance mutations (TDRM) are detected. For some TDRM it has been shown that they revert to wild type while other mutations persist in the absence of therapy. To understand the mechanisms explaining persistence we investigated the in vivo evolution of frequently transmitted HIV-1 variants and their impact on in vitro replicative capacity.
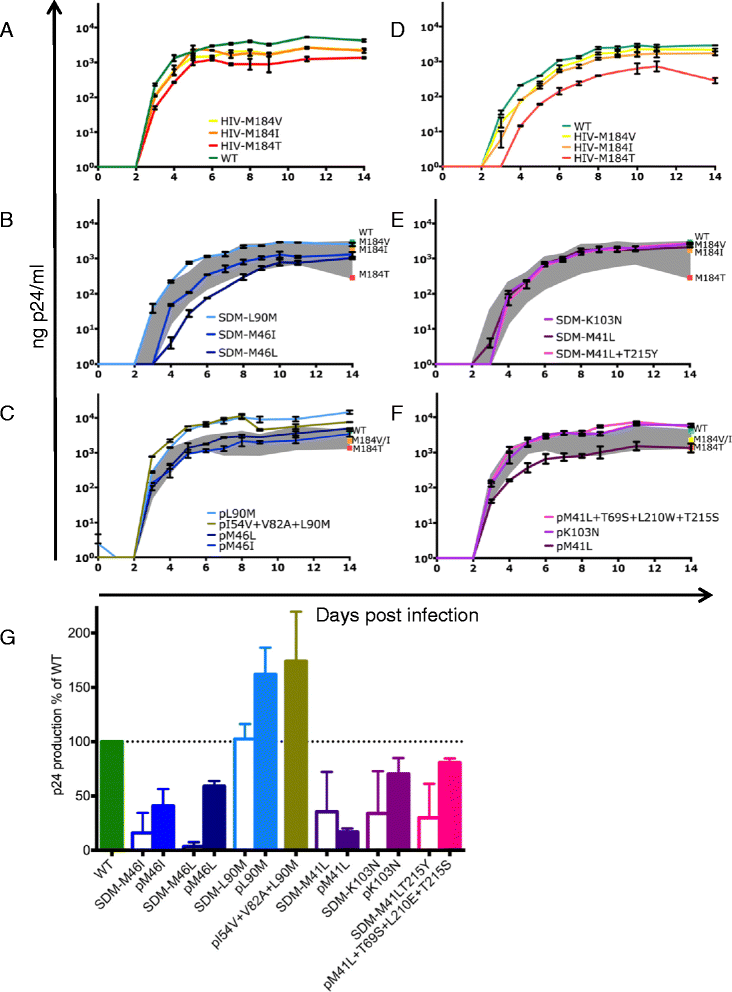
Results: We selected 31 individuals infected with HIV-1 harboring frequently observed TDRM such as M41L or K103N in reverse transcriptase (RT) or M46L in protease. In all these samples, polymorphisms at non-TDRM positions were present at baseline (median protease: 5, RT: 6). Extensive analysis of viral evolution of protease and RT demonstrated that the majority of TDRM (51/55) persisted for at least a year and even up to eight years in the plasma. During follow-up only limited selection of additional polymorphisms was observed (median: 1).To investigate the impact of frequently observed TDRM on the replication capacity, mutant viruses were constructed with the most frequently encountered TDRM as site-directed mutants in the genetic background of the lab strain HXB2. In addition, viruses containing patient-derived protease or RT harboring similar TDRM were made. The replicative capacity of all viral variants was determined by infecting peripheral blood mononuclear cells and subsequently monitoring virus replication. The majority of site-directed mutations (M46I/M46L in protease and M41L, M41L + T215Y and K103N in RT) decreased viral replicative capacity; only protease mutation L90M did not hamper viral replication. Interestingly, most patient-derived viruses had a higher in vitro replicative capacity than the corresponding site-directed mutant viruses.
Conclusions: We demonstrate limited in vivo evolution of protease and RT harbouring frequently observed TDRM in the plasma. This is in line with the high in vitro replication capacity of patient-derived viruses harbouring TDRM compared to site-directed mutant viruses harbouring TDRM. As site-directed mutant viruses have a lower replication capacity than the patient-derived viruses with similar mutational patterns, we propose that (baseline) polymorphisms function as compensatory mutations improving viral replication capacity.
Figures
References
-
- Wheeler WH, Ziebell RA, Zabina H, Pieniazek D, Prejean J, Bodnar UR, Mahle KC, Heneine W, Johnson JA, Hall HI. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U.S.-2006. AIDS. 2010;24:1203–1212. doi: 10.1097/QAD.0b013e3283388742. - DOI - PubMed
-
- Vercauteren J, Wensing AM, van de Vijver DA, Albert J, Balotta C, Hamouda O, Kucherer C, Struck D, Schmit JC, Asjo B, Bruckova M, Camacho RJ, Clotet B, Coughlan S, Grossman Z, Horban A, Korn K, Kostrikis L, Nielsen C, Paraskevis D, Poljak M, Puchhammer-Stöckl E, Riva C, Ruiz L, Salminen M, Schuurman R, Sonnerborg A, Stanekova D, Stanojevic M, Vandamme AM, et al. Transmission of drug-resistant HIV-1 is stabilizing in Europe. J Infect Dis. 2009;200:1503–1508. doi: 10.1086/644505. - DOI - PubMed
-
- SPREAD programme . 9th European Workshop on HIV & Hepatitis Treatment Strategies & Antiviral Drug Resistance. Volume 2. Paphos, Cyprus: Reviews in Antiviral Therapy & Infectious Diseases; 2011. Recent dynamics of transmitted drug resistance in Europe: SPREAD programme 2006–2007; pp. 15–16.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

