Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study
- PMID: 25575253
- PMCID: PMC5074683
- DOI: 10.1097/SLA.0000000000000993
Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study
Abstract
Objective: The primary aim of this trial was to assess the feasibility of minimally invasive esophagectomy (MIE) in a multi-institutional setting.
Background: Esophagectomy is an important, potentially curative treatment for localized esophageal cancer, but is a complex operation. MIE may decrease the morbidity and mortality of resection, and single-institution studies have demonstrated successful outcomes with MIE.
Methods: We conducted a multicenter, phase II, prospective, cooperative group study (coordinated by the Eastern Cooperative Oncology Group) to evaluate the feasibility of MIE. Patients with biopsy-proven high-grade dysplasia or esophageal cancer were enrolled at 17 credentialed sites. Protocol surgery consisted of either 3-stage MIE or Ivor Lewis MIE. The primary end point was 30-day mortality. Secondary end points included adverse events, duration of hospital-stay, and 3-year outcomes.
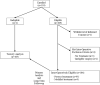
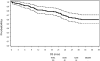
Results: Protocol surgery was completed in 95 of the 104 patients eligible for the primary analysis (91.3%). The 30-day mortality in eligible patients who underwent MIE was 2.1%; perioperative mortality in all registered patients eligible for primary analysis was 2.9%. Median intensive care unit and hospital stay were 2 and 9 days, respectively. Grade 3 or higher adverse events included anastomotic leak (8.6%), acute respiratory distress syndrome (5.7%), pneumonitis (3.8%), and atrial fibrillation (2.9%). At a median follow-up of 35.8 months, the estimated 3-year overall survival was 58.4% (95% confidence interval: 47.7%-67.6%). Locoregional recurrence occurred in only 7 patients (6.7%).
Conclusions: This prospective multicenter study demonstrated that MIE is feasible and safe with low perioperative morbidity and mortality and good oncological results. This approach can be adopted by other centers with appropriate expertise in open esophagectomy and minimally invasive surgery.
Conflict of interest statement
The authors have no relevant conflicts of interest related to this manuscript.
Figures

Comment in
-
[Minimally invasive esophagus resection: Results of a prospective multicenter study].Chirurg. 2015 Sep;86(9):898. doi: 10.1007/s00104-015-0067-z. Chirurg. 2015. PMID: 26223670 German. No abstract available.
References
-
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–52. - PubMed
-
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12. - PubMed
-
- Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. - PubMed
Publication types
MeSH terms
Grants and funding
- U10 CA032291/CA/NCI NIH HHS/United States
- CA32291/CA/NCI NIH HHS/United States
- CA66636/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA017145/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA039229/CA/NCI NIH HHS/United States
- CA39229/CA/NCI NIH HHS/United States
- CA17145/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
- UG1 CA233184/CA/NCI NIH HHS/United States

