Abnormal accumulation of desmin in gastrocnemius myofibers of patients with peripheral artery disease: associations with altered myofiber morphology and density, mitochondrial dysfunction and impaired limb function
- PMID: 25575565
- PMCID: PMC4374059
- DOI: 10.1369/0022155415569348
Abnormal accumulation of desmin in gastrocnemius myofibers of patients with peripheral artery disease: associations with altered myofiber morphology and density, mitochondrial dysfunction and impaired limb function
Abstract
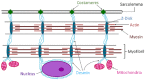
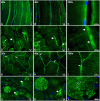
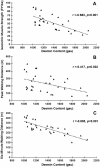
Patients with peripheral artery disease (PAD) develop a myopathy in their ischemic lower extremities, which is characterized by myofiber degeneration, mitochondrial dysfunction and impaired limb function. Desmin, a protein of the cytoskeleton, is central to maintenance of the structure, shape and function of the myofiber and its organelles, especially the mitochondria, and to translation of sarcomere contraction into muscle contraction. In this study, we investigated the hypothesis that disruption of the desmin network occurs in gastrocnemius myofibers of PAD patients and correlates with altered myofiber morphology, mitochondrial dysfunction, and impaired limb function. Using fluorescence microscopy, we evaluated desmin organization and quantified myofiber content in the gastrocnemius of PAD and control patients. Desmin was highly disorganized in PAD but not control muscles and myofiber content was increased significantly in PAD compared to control muscles. By qPCR, we found that desmin gene transcripts were increased in the gastrocnemius of PAD patients as compared with control patients. Increased desmin and desmin gene transcripts in PAD muscles correlated with altered myofiber morphology, decreased mitochondrial respiration, reduced calf muscle strength and decreased walking performance. In conclusion, our studies identified disruption of the desmin system in gastrocnemius myofibers as an index of the myopathy and limitation of muscle function in patients with PAD.
Keywords: Cytoskeleton; desmin; intermittent claudication; muscle disease; myofiber.
© The Author(s) 2015.
Conflict of interest statement
Figures



References
-
- Abraham SC, DeNofrio D, Loh E, Minda JM, Tomaszewski JE, Pietra GG, Reynolds C. (1998). Desmin myopathy involving cardiac, skeletal, and vascular smooth muscle: report of a case with immunoelectron microscopy. Hum Pathol 29:876-882. - PubMed
-
- Anderson JD, Epstein FH, Meyer CH, Hagspiel KD, Wang H, Berr SS, Harthun NL, Weltman A, Dimaria JM, West AM, Kramer CM. (2009). Multifactorial determinants of functional capacity in peripheral arterial disease: uncoupling of calf muscle perfusion and metabolism. J Am Coll Cardiol 54:628-635. - PMC - PubMed
-
- Bloch RJ, Gonzalez-Serratos H. (2003). Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev 31:73-78. - PubMed
-
- Brass EP. (1996). Skeletal muscle metabolism as a target for drug therapy in peripheral arterial disease. Vasc Med 1:55-59. - PubMed
-
- Brass EP, Hiatt WR. (2000). Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc Med 5:55-59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

