Personalized contact strategies and predictors of time to survey completion: analysis of two sequential randomized trials
- PMID: 25575599
- PMCID: PMC4407535
- DOI: 10.1186/1471-2288-15-5
Personalized contact strategies and predictors of time to survey completion: analysis of two sequential randomized trials
Abstract
Background: Effective strategies for contacting and recruiting study participants are critical in conducting clinical research. In this study, we conducted two sequential randomized controlled trials of mail- and telephone-based strategies for contacting and recruiting participants, and evaluated participant-related variables' association with time to survey completion and survey completion rates. Subjects eligible for this study were survivors of acute lung injury who had been previously enrolled in a 12-month observational follow-up study evaluating their physical, cognitive and mental health outcomes, with their last study visit completed at a median of 34 months previously.
Methods: Eligible subjects were contacted to complete a new research survey as part of two randomized trials, initially using a randomized mail-based contact strategy, followed by a randomized telephone-based contact strategy for non-responders to the mail strategy. Both strategies focused on using either a personalized versus a generic approach. In addition, 18 potentially relevant subject-related variables (e.g., demographics, last known physical and mental health status) were evaluated for association with time to survey completion.
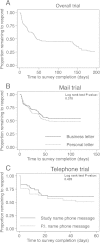
Results: Of 308 eligible subjects, 67% completed the survey with a median (IQR) of 3 (2, 5) contact attempts required. There was no significant difference in the time to survey completion for either randomized trial of mail- or phone-based contact strategy. Among all subject-related variables, age ≤40 years and minority race were independently associated with a longer time to survey completion.
Conclusion: We found that age ≤40 years and minority race were associated with a longer time to survey completion, but personalized versus generic approaches to mail- and telephone-based contact strategies had no significant effect. Repeating both mail and telephone contact attempts was important for increasing survey completion rate.
Trial registration: NCT00719446.
Figures


References
-
- Treweek S, Pitkethly M, Cook J, Kjeldstrom M, Taskila T, Johansen M, et al. Cochrane Database Syst Rev. 2010. Strategies to improve recruitment to randomised controlled trials. - PubMed
-
- Puffer S, Torgerson DJ. Recruitment difficulties in randomised controlled trials. Control Clin Trials. 2003;14:214s–215s.
Pre-publication history
-
- The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2288/15/5/prepub
Publication types
MeSH terms
Associated data
Grants and funding
- HHSN268200536172C/PHS HHS/United States
- 3R01HL091760-02S1/HL/NHLBI NIH HHS/United States
- HHSN268200536171C/PHS HHS/United States
- HHSN268200536165C/PHS HHS/United States
- HHSN268200536170C/PHS HHS/United States
- R01 HL088045/HL/NHLBI NIH HHS/United States
- HHSN268200536174C/PHS HHS/United States
- 1R24HL111895/HL/NHLBI NIH HHS/United States
- N01 HR056170/HL/NHLBI NIH HHS/United States
- HHSN268200536173C/PHS HHS/United States
- HHSN268200536169C/PHS HHS/United States
- HHSN268200536176C/PHS HHS/United States
- HHSN268200536179C/PHS HHS/United States
- HHSN268200536168C/PHS HHS/United States
- HHSN268200536167C/PHS HHS/United States
- HHSN268200536175C/PHS HHS/United States
- R01HL091760/HL/NHLBI NIH HHS/United States
- R01 HL091760/HL/NHLBI NIH HHS/United States
- R24 HL111895/HL/NHLBI NIH HHS/United States
- HHSN268200536166C/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

