Myoconductive and osteoinductive free-standing polysaccharide membranes
- PMID: 25575853
- PMCID: PMC4540078
- DOI: 10.1016/j.actbio.2014.12.027
Myoconductive and osteoinductive free-standing polysaccharide membranes
Abstract
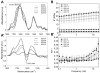
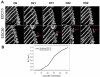
Free-standing (FS) membranes have increasing applications in the biomedical field as drug delivery systems for wound healing and tissue engineering. Here, we studied the potential of free-standing membranes made by the layer-by-layer assembly of chitosan and alginate to be used as a simple biomimetic system of the periosteum. The design of a periosteum-like membrane implies the elaboration of a thick membrane suitable for both muscle and bone formation. Our aim was to produce well-defined ∼50 μm thick polysaccharide membranes that could be easily manipulated, were mechanically resistant, and would enable both myogenesis and osteogenesis in vitro and in vivo. The membranes were chemically crosslinked to improve their mechanical properties. Crosslinking chemistry was followed via Fourier transform infrared spectroscopy and the mechanical properties of the membranes were assessed using dynamic mechanical analysis. The loading and release of the potent osteoinductive growth factor bone morphogenetic protein 2 (BMP-2) inside and outside of the FS membrane was followed by fluorescence spectroscopy in a physiological buffer over 1 month. The myogenic and osteogenic potentials of the membranes in vitro were assessed using BMP-2-responsive skeletal myoblasts. Finally, their osteoinductive properties in vivo were studied in a preliminary experiment using a mouse ectopic model. Our results showed that the more crosslinked FS membranes enabled a more efficient myoblast differentiation in myotubes. In addition, we showed that a tunable amount of BMP-2 can be loaded into and subsequently released from the membranes, depending on the crosslinking degree and the initial BMP-2 concentration in solution. Only the more crosslinked membranes were found to be osteoinductive in vivo. These polysaccharide-based membranes have strong potential as a periosteum-mimetic scaffold for bone tissue regeneration.
Keywords: Biomaterials; Free-standing membranes; Layer-by-layer; Osteoinduction; Polysaccharides.
Copyright © 2015 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures





References
-
- Paulsson M. Basement Membrane Proteins: Structure, Assembly, and Cellular Interactions. Crit Rev Biochem Mol. 1992;27:93–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

