Distinct functional roles of cytoplasmic dynein defined by the intermediate chain isoforms
- PMID: 25576383
- PMCID: PMC4433767
- DOI: 10.1016/j.yexcr.2014.12.013
Distinct functional roles of cytoplasmic dynein defined by the intermediate chain isoforms
Abstract
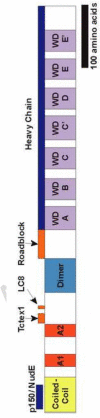
The motor protein, cytoplasmic dynein is responsible for the movement of a variety of cargoes toward microtubule minus ends in cells. Little is understood about how dynein is regulated to specifically transport its various cargoes. In vertebrates, the dynein motor domain (DYNC1H) is encoded by a single gene; while there are two genes for the five smaller subunits that comprise the cargo binding domain of the dynein complex. The isoforms of the intermediate chain (DYNC1I) provide a good model system with which to study the roles the different isoforms of the cargo domain subunits have in designating specific dynein functions. The intermediate chains (DYNC1I) play a key scaffold role in the dynein complex. In neurons, dynein complexes with different intermediate chain isoforms have distinct roles, including cargo binding and transport. Some of the phospho-isoforms of the intermediate chain also specify binding to specific cargo. These data support the model that cytoplasmic dynein can be specifically regulated through the different isoforms of the subunits.
Keywords: Axonal transport; Cytoplasmic dynein; Cytoskeleton; Intermediate chain phosphorylation; Motor protein.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

References
-
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. - PubMed
-
- Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: An ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58:189–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

