Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy
- PMID: 25576433
- PMCID: PMC4381099
- DOI: 10.1007/s00520-014-2597-1
Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy
Abstract
Purpose: Pegfilgrastim is a pegylated form of filgrastim, a recombinant protein of granulocyte colony-stimulating factor, that is used to reduce the risk of febrile neutropenia (FN). Here, we report the results of a phase III trial of pegfilgrastim in breast cancer patients receiving docetaxel and cyclophosphamide (TC) chemotherapy.
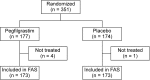
Methods: We conducted a double-blind, placebo-controlled, randomized trial to determine the efficacy of pegfilgrastim in reducing the risk of FN in early-stage breast cancer patients. A total of 351 women (177 in the pegfilgrastim group and 174 in the placebo group) between 20 and 69 years of age with stage I-III invasive breast carcinoma who were to receive TC chemotherapy (docetaxel 75 mg/m(2) and cyclophosphamide 600 mg/m(2) every 3 weeks) as either neoadjuvant or adjuvant therapy were enrolled; 346 of these patients were treated with either pegfilgrastim (n = 173) or placebo (n = 173).
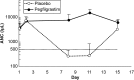
Results: The incidence of FN was significantly lower in the pegfilgrastim group than in the placebo group (1.2 vs. 68.8 %, respectively; P < 0.001). In addition, patients in the pegfilgrastim group required less hospitalization and antibiotics for FN. Most adverse events were consistent with those expected for breast cancer subjects receiving TC chemotherapy.
Conclusions: Pegfilgrastim is safe and significantly reduces the incidence of FN in breast cancer patients.
Figures
References
-
- Blackwell S, Crawford J. Filgrastim (r-metHuG-CSF) in the chemotherapy setting. In: Morstyn G, Dexter TM, editors. Filgrastim (r-metHuG-CSF) in Clinical Practice. New York: Marcel Dekker, Inc.; 1994. pp. 103–116.
-
- Bosly A, Bron D, van Hoof A, de Bock R, Berneman Z, Ferrant A, Kaufman L, Dauwe M, Verhoef G. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol. 2008;87:277–283. doi: 10.1007/s00277-007-0399-y. - DOI - PubMed
-
- Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. - DOI - PubMed
-
- National Comprehensive Cancer Network (2014) Clinical practice guidelines in oncology: myeloid growth factors, version 1. National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/pdf/myeloid_growth.pdf. Accessed 6 June 2014 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

