Covalent adduct formation between the plasmalogen-derived modification product 2-chlorohexadecanal and phloretin
- PMID: 25576489
- PMCID: PMC4321883
- DOI: 10.1016/j.bcp.2014.12.017
Covalent adduct formation between the plasmalogen-derived modification product 2-chlorohexadecanal and phloretin
Abstract
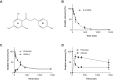
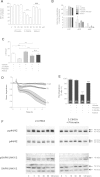
Hypochlorous acid added as reagent or generated by the myeloperoxidase (MPO)-H2O2-Cl(-) system oxidatively modifies brain ether-phospholipids (plasmalogens). This reaction generates a sn2-acyl-lysophospholipid and chlorinated fatty aldehydes. 2-Chlorohexadecanal (2-ClHDA), a prototypic member of chlorinated long-chain fatty aldehydes, has potent neurotoxic potential by inflicting blood-brain barrier (BBB) damage. During earlier studies we could show that the dihydrochalcone-type polyphenol phloretin attenuated 2-ClHDA-induced BBB dysfunction. To clarify the underlying mechanism(s) we now investigated the possibility of covalent adduct formation between 2-ClHDA and phloretin. Coincubation of 2-ClHDA and phloretin in phosphatidylcholine liposomes revealed a half-life of 2-ClHDA of approx. 120min, decaying at a rate of 5.9×10(-3)min(-1). NMR studies and enthalpy calculations suggested that 2-ClHDA-phloretin adduct formation occurs via electrophilic aromatic substitution followed by hemiacetal formation on the A-ring of phloretin. Adduct characterization by high-resolution mass spectroscopy confirmed these results. In contrast to 2-ClHDA, the covalent 2-ClHDA-phloretin adduct was without adverse effects on MTT reduction (an indicator for metabolic activity), cellular adenine nucleotide content, and barrier function of brain microvascular endothelial cells (BMVEC). Of note, 2-ClHDA-phloretin adduct formation was also observed in BMVEC cultures. Intraperitoneal application and subsequent GC-MS analysis of brain lipid extracts revealed that phloretin is able to penetrate the BBB of C57BL/6J mice. Data of the present study indicate that phloretin scavenges 2-ClHDA, thereby attenuating 2-ClHDA-mediated brain endothelial cell dysfunction. We here identify a detoxification pathway for a prototypic chlorinated fatty aldehyde (generated via the MPO axis) that compromises BBB function in vitro and in vivo.
Keywords: Blood–brain barrier; Chlorinated fatty aldehyde; Myeloperoxidase; Neuroinflammation; Plasmalogens.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Zlokovic B.V. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. - PubMed
-
- Hawkins B.T., Davis T.P. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. - PubMed
-
- Coisne C., Engelhardt B. Tight junctions in brain barriers during central nervous system inflammation. Antioxid Redox Signal. 2011;15:1285–1303. - PubMed
-
- Blasig I.E., Bellmann C., Cording J., Del Vecchio G., Zwanziger D., Huber O. Occludin protein family: oxidative stress and reducing conditions. Antioxid Redox Signal. 2011;15:1195–1219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

