Envisioning the dynamics and flexibility of Mre11-Rad50-Nbs1 complex to decipher its roles in DNA replication and repair
- PMID: 25576492
- PMCID: PMC4417436
- DOI: 10.1016/j.pbiomolbio.2014.12.004
Envisioning the dynamics and flexibility of Mre11-Rad50-Nbs1 complex to decipher its roles in DNA replication and repair
Abstract
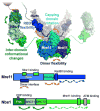
The Mre11-Rad50-Nbs1 (MRN) complex is a dynamic macromolecular machine that acts in the first steps of DNA double strand break repair, and each of its components has intrinsic dynamics and flexibility properties that are directly linked with their functions. As a result, deciphering the functional structural biology of the MRN complex is driving novel and integrated technologies to define the dynamic structural biology of protein machinery interacting with DNA. Rad50 promotes dramatic long-range allostery through its coiled-coil and zinc-hook domains. Its ATPase activity drives dynamic transitions between monomeric and dimeric forms that can be modulated with mutants modifying the ATPase rate to control end joining versus resection activities. The biological functions of Mre11's dual endo- and exonuclease activities in repair pathway choice were enigmatic until recently, when they were unveiled by the development of specific nuclease inhibitors. Mre11 dimer flexibility, which may be regulated in cells to control MRN function, suggests new inhibitor design strategies for cancer intervention. Nbs1 has FHA and BRCT domains to bind multiple interaction partners that further regulate MRN. One of them, CtIP, modulates the Mre11 excision activity for homologous recombination repair. Overall, these combined properties suggest novel therapeutic strategies. Furthermore, they collectively help to explain how MRN regulates DNA repair pathway choice with implications for improving the design and analysis of cancer clinical trials that employ DNA damaging agents or target the DNA damage response.
Keywords: Allostery; Conformational change; CtIP; Double strand break repair; Dynamics; Mre11-Rad50-Nbs1.
Published by Elsevier Ltd.
Figures


References
-
- Al-Ahmadie H, Iyer G, Hohl M, Asthana S, Inagaki A, Schultz N, Hanrahan AJ, Scott SN, Brannon AR, McDermott GC, Pirun M, Ostrovnaya I, Kim P, Socci ND, Viale A, Schwartz GK, Reuter V, Bochner BH, Rosenberg JE, Bajorin DF, Berger MF, Petrini JHJ, Solit DB, Taylor BS. Synthetic Lethality in ATM-Deficient RAD50-Mutant Tumors Underlies Outlier Response to Cancer Therapy. Cancer Discov. 2014;4:1014–1021. doi: 10.1158/2159-8290.CD-14-0380. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

