Microbial engineering for aldehyde synthesis
- PMID: 25576610
- PMCID: PMC4345389
- DOI: 10.1128/AEM.03319-14
Microbial engineering for aldehyde synthesis
Abstract
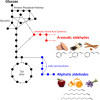
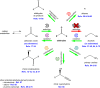
Aldehydes are a class of chemicals with many industrial uses. Several aldehydes are responsible for flavors and fragrances present in plants, but aldehydes are not known to accumulate in most natural microorganisms. In many cases, microbial production of aldehydes presents an attractive alternative to extraction from plants or chemical synthesis. During the past 2 decades, a variety of aldehyde biosynthetic enzymes have undergone detailed characterization. Although metabolic pathways that result in alcohol synthesis via aldehyde intermediates were long known, only recent investigations in model microbes such as Escherichia coli have succeeded in minimizing the rapid endogenous conversion of aldehydes into their corresponding alcohols. Such efforts have provided a foundation for microbial aldehyde synthesis and broader utilization of aldehydes as intermediates for other synthetically challenging biochemical classes. However, aldehyde toxicity imposes a practical limit on achievable aldehyde titers and remains an issue of academic and commercial interest. In this minireview, we summarize published efforts of microbial engineering for aldehyde synthesis, with an emphasis on de novo synthesis, engineered aldehyde accumulation in E. coli, and the challenge of aldehyde toxicity.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Crosland MP. 2004. Historical studies in the language of chemistry. Dover Publications, Mineola, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

