Predicting the impact of adverse events and treatment duration on medical resource utilization-related costs in hepatitis C genotype 1 treatment-naïve patients receiving antiviral therapy
- PMID: 25577042
- PMCID: PMC4381112
- DOI: 10.1007/s40273-014-0249-4
Predicting the impact of adverse events and treatment duration on medical resource utilization-related costs in hepatitis C genotype 1 treatment-naïve patients receiving antiviral therapy
Abstract
Objectives: Studies on medical resource utilization (MRU) and related costs are important for evaluating the potential patient management and cost-effectiveness implications of antiviral treatments for hepatitis C virus (HCV) infection. The objectives of this study were (i) to compare the MRU and related costs for two treatment approaches; (ii) to identify the main drivers of resource use and costs; and (iii) to assess the effects of various treatment regimen attributes on MRU-related costs in a UK clinical setting.
Methods: The analysis used data collected alongside the simeprevir (SMV) phase III trials for treatment-naïve genotype 1 HCV-infected patients; these data covered outpatient consultations with specialists, emergency room visits and hospital admissions. Logistic regressions were constructed to estimate the predictors of resource utilization, and a two-part multivariable analysis model was used to determine the total costs of treatment in the UK.
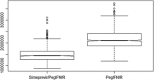
Results: Data on 731 patients receiving SMV plus pegylated interferon and ribavirin (SMV/PegIFN/R) or PegIFN/R were included in the analysis. While MRU was similar between the SMV and PegIFN/R groups, MRU-related costs were significantly lower in the SMV group than in the PegIFN/R group (P < 0.05). High body mass index (P < 0.05), severe fibrosis (P < 0.05), shortened treatment duration to 24 weeks (P < 0.05), and anaemia and rash during treatment (P < 0.001) were identified as predictors of hospitalization and outpatient visits and as drivers of total costs. Univariate sensitivity analyses suggested that shortened treatment duration and lower occurrence of rash lead to large cost savings.
Conclusion: This study identified both baseline and on-treatment antiviral therapy characteristics as drivers of MRU-related costs for HCV patients following antiviral therapy. The shortened treatment duration and reduction in rash due to treatment with SMV triple therapy lead to substantial non-drug cost savings, compared with PegIFN/R treatment. This suggests that there are potential patient management and cost-effectiveness implications associated with the choice of specific antiviral treatments.
Figures

Similar articles
-
Determinant Factors of the Direct Medical Costs Associated with Genotype 1 Hepatitis C Infection in Treatment-Experienced Patients.Drugs R D. 2015 Dec;15(4):335-49. doi: 10.1007/s40268-015-0109-5. Drugs R D. 2015. PMID: 26416653 Free PMC article. Clinical Trial.
-
A cost utility analysis of simeprevir used with peginterferon + ribavirin in the management of genotype 1 hepatitis C virus infection, from the perspective of the UK National Health Service.J Med Econ. 2015;18(10):838-49. doi: 10.3111/13696998.2015.1044457. Epub 2015 Jul 6. J Med Econ. 2015. PMID: 25903830
-
Cost-effectiveness analysis of simeprevir in combination with peginterferon and ribavirin for treatment-naïve chronic hepatitis C genotype 1 patients in Japan.J Med Econ. 2015;18(7):502-11. doi: 10.3111/13696998.2015.1029492. Epub 2015 Mar 31. J Med Econ. 2015. PMID: 25763827
-
Financial impact of two different ways of evaluating early virological response to peginterferon-alpha-2b plus ribavirin therapy in treatment-naive patients with chronic hepatitis C virus genotype 1.Pharmacoeconomics. 2005;23(10):1043-55. doi: 10.2165/00019053-200523100-00007. Pharmacoeconomics. 2005. PMID: 16235977 Review.
-
Efficacy and safety of simeprevir in combination with peginterferon and ribavirin for patients with hepatitis C genotype 1 infection: a meta-analysis of randomized trials.Rev Esp Enferm Dig. 2015 Oct;107(10):591-7. doi: 10.17235/reed.2015.3840/2015. Rev Esp Enferm Dig. 2015. PMID: 26437977 Review.
Cited by
-
Determinant Factors of the Direct Medical Costs Associated with Genotype 1 Hepatitis C Infection in Treatment-Experienced Patients.Drugs R D. 2015 Dec;15(4):335-49. doi: 10.1007/s40268-015-0109-5. Drugs R D. 2015. PMID: 26416653 Free PMC article. Clinical Trial.
References
-
- Costella A, Goldberg D, Harris H, Hutchinson S, Lyons M, McCartney M, et al. Hepatitis C in the UK: 2013 report. London: Public Health England; 2013.
-
- Sánchez Á. Module XIX cost efficacy and cost–benefit of treatment of hepatitis C. Ann Hepatol. 2006;5(Suppl 1):S69–S73.
-
- Backx M, Lewszuk A, White JR, Cole J, Sreedharan A, van Sanden S, et al. The cost of treatment failure: resource use and costs incurred by hepatitis C virus genotype 1-infected patients who do or do not achieve sustained virological response to therapy. J Viral Hepat. 2014;21(3):208–215. doi: 10.1111/jvh.12132. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

