Phase I study of nanoliposomal irinotecan (PEP02) in advanced solid tumor patients
- PMID: 25577133
- PMCID: PMC4341010
- DOI: 10.1007/s00280-014-2671-x
Phase I study of nanoliposomal irinotecan (PEP02) in advanced solid tumor patients
Abstract
Purpose: To define the dose-limiting toxicity (DLT), maximum tolerated dose (MTD) and pharmacokinetics (PK) of PEP02, a novel liposome-encapsulated irinotecan, in patients with advanced refractory solid tumors.
Methods: Patients were enrolled in cohorts of one to three to receive escalating dose of PEP02 in a phase I trial. PEP02, from 60 to 180 mg/m(2), was given as a 90-min intravenous infusion, every 3 weeks.
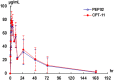
Results: A total of 11 patients were enrolled into three dose levels: 60 (one patient), 120 (six patients) and 180 mg/m(2) (four patients). DLT was observed in three patients, one at 120 mg/m(2) (grade 3 catheter-related infection) and two at 180 mg/m(2) (grade 4 neutropenia lasting for >3 days in one, grade 4 hematological toxicities and grade 4 diarrhea in the other). MTD was determined as 120 mg/m(2). Comparing with those after free-form irinotecan in the literature, the dose-normalized PK of SN-38 (the active metabolite) after PEP02 was characterized by lower C max, prolonged terminal half-life and higher AUC but with significant inter-individual variation. One patient who died of treatment-related toxicity had significantly higher C max and AUC levels of SN-38 than those of the other three patients at 180 mg/m(2). Post hoc pharmacogenetic study showed that the patient had a combined heterozygosity genotype of UGT1A1*6/*28. Two patients had objective tumor response.
Conclusions: PEP02 apparently modified the PK parameters of irinotecan and SN-38 by liposome encapsulation. The MTD of PEP02 monotherapy at 3-week interval is 120 mg/m(2), which will be the recommended dose for future studies.
Figures


References
-
- Iyer L, King CD, Whitington PF, Green MD, Roy SK, Tephly TR, Coffman BL, Ratain MJ. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest. 1998;101:847–854. doi: 10.1172/JCI915. - DOI - PMC - PubMed
-
- Zamboni WC, Jung LL, Egorin MJ, Hamburger DR, Joseph E, Jin R, Strychor S, Ramanathan RK, Eiseman JL. Relationship between plasma exposure of 9-nitrocamptothecin and its 9-aminocamptothecin metabolite and antitumor response in mice bearing human colon carcinoma xenografts. Clin Cancer Res. 2005;11:4867–4874. doi: 10.1158/1078-0432.CCR-05-0144. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

