Improved approach for chondrogenic differentiation of human induced pluripotent stem cells
- PMID: 25578634
- PMCID: PMC4412587
- DOI: 10.1007/s12015-014-9581-5
Improved approach for chondrogenic differentiation of human induced pluripotent stem cells
Abstract
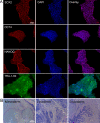
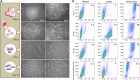
Human induced pluripotent stem cells (hiPSCs) have demonstrated great potential for hyaline cartilage regeneration. However, current approaches for chondrogenic differentiation of hiPSCs are complicated and inefficient primarily due to intermediate embryoid body formation, which is required to generate endodermal, ectodermal, and mesodermal cell lineages. We report a new, straightforward and highly efficient approach for chondrogenic differentiation of hiPSCs, which avoids embryoid body formation. We differentiated hiPSCs directly into mesenchymal stem /stromal cells (MSC) and chondrocytes. hiPSC-MSC-derived chondrocytes showed significantly increased Col2A1, GAG, and SOX9 gene expression compared to hiPSC-MSCs. Following transplantation of hiPSC-MSC and hiPSC-MSC-derived chondrocytes into osteochondral defects of arthritic joints of athymic rats, magnetic resonance imaging studies showed gradual engraftment, and histological correlations demonstrated hyaline cartilage matrix production. Results present an efficient and clinically translatable approach for cartilage tissue regeneration via patient-derived hiPSCs, which could improve cartilage regeneration outcomes in arthritic joints.
Figures




Similar articles
-
Cord blood cell-derived iPSCs as a new candidate for chondrogenic differentiation and cartilage regeneration.Stem Cell Res Ther. 2017 Jan 28;8(1):16. doi: 10.1186/s13287-017-0477-6. Stem Cell Res Ther. 2017. PMID: 28129782 Free PMC article.
-
Repair potential of nonsurgically delivered induced pluripotent stem cell-derived chondrocytes in a rat osteochondral defect model.J Tissue Eng Regen Med. 2018 Aug;12(8):1843-1855. doi: 10.1002/term.2705. Epub 2018 Jul 2. J Tissue Eng Regen Med. 2018. PMID: 29770595
-
Comparison of Four Protocols to Generate Chondrocyte-Like Cells from Human Induced Pluripotent Stem Cells (hiPSCs).Stem Cell Rev Rep. 2017 Apr;13(2):299-308. doi: 10.1007/s12015-016-9708-y. Stem Cell Rev Rep. 2017. PMID: 27987073 Free PMC article.
-
The use of mesenchymal stem cells for chondrogenesis.Injury. 2008 Apr;39 Suppl 1:S58-65. doi: 10.1016/j.injury.2008.01.038. Injury. 2008. PMID: 18313473 Review.
-
Small Molecule Regulation of Stem Cells that Generate Bone, Chondrocyte, and Cardiac Cells.Curr Top Med Chem. 2020;20(26):2344-2361. doi: 10.2174/1568026620666200820143912. Curr Top Med Chem. 2020. PMID: 32819246 Review.
Cited by
-
Versatility of Induced Pluripotent Stem Cells (iPSCs) for Improving the Knowledge on Musculoskeletal Diseases.Int J Mol Sci. 2020 Aug 25;21(17):6124. doi: 10.3390/ijms21176124. Int J Mol Sci. 2020. PMID: 32854405 Free PMC article. Review.
-
Different Chondrogenic Potential among Human Induced Pluripotent Stem Cells from Diverse Origin Primary Cells.Stem Cells Int. 2018 Jan 21;2018:9432616. doi: 10.1155/2018/9432616. eCollection 2018. Stem Cells Int. 2018. PMID: 29535785 Free PMC article.
-
Articular cartilage tissue engineering: the role of signaling molecules.Cell Mol Life Sci. 2016 Mar;73(6):1173-94. doi: 10.1007/s00018-015-2115-8. Epub 2016 Jan 25. Cell Mol Life Sci. 2016. PMID: 26811234 Free PMC article. Review.
-
Cord blood cell-derived iPSCs as a new candidate for chondrogenic differentiation and cartilage regeneration.Stem Cell Res Ther. 2017 Jan 28;8(1):16. doi: 10.1186/s13287-017-0477-6. Stem Cell Res Ther. 2017. PMID: 28129782 Free PMC article.
-
The Role of MicroRNAs in Early Chondrogenesis of Human Induced Pluripotent Stem Cells (hiPSCs).Int J Mol Sci. 2019 Sep 5;20(18):4371. doi: 10.3390/ijms20184371. Int J Mol Sci. 2019. PMID: 31492046 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

