Hormonal contraceptive use and women's risk of HIV acquisition: a meta-analysis of observational studies
- PMID: 25578825
- PMCID: PMC4526270
- DOI: 10.1016/S1473-3099(14)71052-7
Hormonal contraceptive use and women's risk of HIV acquisition: a meta-analysis of observational studies
Abstract
Background: The evidence from epidemiological research into whether use of hormonal contraception increases women's risk of HIV acquisition is inconsistent. We did a robust meta-analysis of existing data to provide summary estimates by hormonal contraceptive method which can be used to inform contraceptive guidelines, models, and future studies.
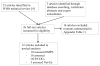
Methods: We updated a recent systematic review to identify and describe studies that met inclusion criteria. To ensure inclusion of more recent research, we searched PubMed for articles published after December, 2011, using the terms "hormonal contraception", "HIV/acquisition", "injectables", "progestin", and "oral contraceptive pills". We assessed statistical heterogeneity for these studies, and, when appropriate, combined point estimates by hormonal contraception formulation using random-effects models. We assessed publication bias and investigated heterogeneity through subgroup and stratified analyses according to study population and design features.
Findings: We identified 26 studies, 12 of which met inclusion criteria. There was evidence of an increase in HIV risk in the ten studies of depot medroxyprogesterone acetate (pooled hazard ratio [HR] 1·40, 95% CI 1·16-1·69). This risk was lower in the eight studies done in women in the general population (pooled HR 1·31, 95% CI 1·10-1·57). There was substantial between-study heterogeneity in secondary analyses of trials (n=7, I(2) 51·1%, 95% CI 0-79·3). Although individual study estimates suggested an increased risk, substantial heterogeneity between two studies done in women at high risk of HIV infection (I(2) 54%, 0-88·7) precluded pooling estimates. There was no evidence of an increased HIV risk in ten studies of oral contraceptive pills (pooled HR 1·00, 0·86-1·16) or five studies of norethisterone enanthate (pooled HR 1·10, 0·88-1·37).
Interpretation: Our findings show a moderate increased risk of HIV acquisition for all women using depot medroxyprogesterone acetate, with a smaller increase in risk for women in the general population. Whether the risks of HIV observed in our study would merit complete withdrawal of depot medroxyprogesterone acetate needs to be balanced against the known benefits of a highly effective contraceptive.
Funding: None.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
All authors have no financial or personal conflicts to disclose.
Figures
Comment in
-
Study supports link between injectable hormonal contraceptive and HIV risk.BMJ. 2015 Jan 9;350:h112. doi: 10.1136/bmj.h112. BMJ. 2015. PMID: 25575721 No abstract available.
-
Broadening the debate over HIV and hormonal contraception.Lancet Infect Dis. 2015 Feb;15(2):135-6. doi: 10.1016/S1473-3099(14)71076-X. Epub 2015 Jan 9. Lancet Infect Dis. 2015. PMID: 25578824 Free PMC article. No abstract available.
-
End of the debate on hormonal contraception and HIV risk?Lancet Infect Dis. 2015 Feb;15(2):131. doi: 10.1016/S1473-3099(15)70011-3. Epub 2015 Jan 19. Lancet Infect Dis. 2015. PMID: 25749056 No abstract available.
References
-
- Affairs UNDoEaS. World Contraceptive Use 2011. 2012 Jan 24;2011 Available from: http://www.un.org/esa/population/publications/contraceptive2011/wallchar....
-
- Singh S, Darroch JE, Ashford LS, Vlassoff M. Adding it up: The costs and benefits of investing in family planning and maternal and newborn health. New York: Guttmacher Institute and United Nations Population Fund; 2009.
-
- Hormonal contraception and HIV: technical statement. Geneva, Switzerland: WHO; 2012. - PubMed
-
- Jain AK. Hormonal contraception and HIV acquisition risk: implications for individual users and public policies. Contraception. 2012;86(6):645–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous