Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis
- PMID: 25578980
- PMCID: PMC4289852
- DOI: 10.4081/ejh.2014.2461
Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis
Abstract
Native fluorescence, or autofluorescence (AF), consists in the emission of light in the UV-visible, near-IR spectral range when biological substrates are excited with light at suitable wavelength. This is a well-known phenomenon, and the strict relationship of many endogenous fluorophores with morphofunctional properties of the living systems, influencing their AF emission features, offers an extremely powerful resource for directly monitoring the biological substrate condition. Starting from the last century, the technological progresses in microscopy and spectrofluorometry were convoying attention of the scientific community to this phenomenon. In the future, the interest in the autofluorescence will certainly continue. Current instrumentation and analytical procedures will likely be overcome by the unceasing progress in new devices for AF detection and data interpretation, while a progress is expected in the search and characterization of endogenous fluorophores and their roles as intrinsic biomarkers.
Conflict of interest statement
Conflict of interests: the authors declare no conflicts of interest.
Figures




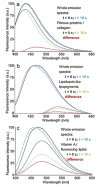
References
-
- Udenfriend S. Fluorescence Assay in Biology and Medicine. Vol II London: Academic Press; 1969.
-
- Kasten FH. The origins of modern fluorescence microscopy and fluorescence probes, p. 4-47 In: Kohen E., Hirschberg J.G. JG (eds.), Cell structure and function by microspectrofluorometry. Academic Press Inc., 1989.
-
- Balaban RS, Mandel LJ. Optical methods for the study of metabolism in intact cells, p. 213-36 In: Forskett J.K., Grinstein S. (eds.), Non-invasive techniques in cell biology. Wiley Liss, 1990.
-
- Rost FWD. Fluorescence microscopy, vol 2 Cambridge University Press, 1995.
-
- Wagnières GA, Star WM, Wilson BC. In vivo fluorescence spectroscopy and imaging for oncological applications. Photochem Photobiol 1998;68:603-32. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

