Inflammatory Chemokines MIP-1δ and MIP-3α Are Involved in the Migration of Multipotent Mesenchymal Stromal Cells Induced by Hepatoma Cells
- PMID: 25579056
- PMCID: PMC4425419
- DOI: 10.1089/scd.2014.0176
Inflammatory Chemokines MIP-1δ and MIP-3α Are Involved in the Migration of Multipotent Mesenchymal Stromal Cells Induced by Hepatoma Cells
Abstract
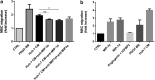
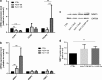
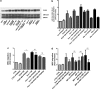
In vivo, bone marrow-derived multipotent mesenchymal stromal cells (MSC) have been identified at sites of tumors, suggesting that specific signals mobilize and activate MSC to migrate to areas surrounding tumors. The signals and migratory mechanisms that guide MSC are not well understood. Here, we investigated the migration of human MSC induced by conditioned medium of Huh-7 hepatoma cells (Huh-7 CM). Using a transwell migration system, we showed that human MSC migration was increased in the presence of Huh-7 CM. Using a human cytokine antibody array, we detected increased levels of MIP-1δ and MIP-3α in Huh-7 CM. Recombinant chemokines MIP-1δ and MIP-3α induced MSC migration. Anti-MIP-1δ and anti-MIP-3α antibodies added to Huh-7 CM decreased MSC migration, further suggesting that MIP-1δ and MIP-3α were implicated in the Huh-7 CM-induced MSC migration. By real-time polymerase chain reaction, we observed an absence of chemokine receptors CCR2 and CXCR2 and low expression of CCR1, CCR5, and CCR6 in MSC. Expression of these chemokine receptors was not regulated by Huh-7 CM. Furthermore, matrix metalloproteinase 1 (MMP-1) expression was strongly increased in MSC after incubation with Huh-7 CM, suggesting that MSC migration depends on MMP-1 activity. The signaling pathway MAPK/ERK was activated by Huh-7 CM but its inhibition by PD98059 did not impair Huh-7 CM-induced MSC migration. Further, long-term incubation of MSC with MIP-1δ increased α-smooth muscle actin expression, suggesting its implication in the Huh-7 CM-induced evolvement of MSC into myofibroblasts. In conclusion, we report that two inflammatory cytokines, MIP-1δ and MIP-3α, are able to increase MSC migration in vitro. These cytokines might be responsible for migration and evolvement of MSC into myofibroblasts around tumors.
Figures




Similar articles
-
Chemokine expression in human erythroid leukemia cell line AS-E2: macrophage inflammatory protein-3alpha/CCL20 is induced by inflammatory cytokines.Exp Hematol. 2006 Jan;34(1):19-26. doi: 10.1016/j.exphem.2005.09.012. Exp Hematol. 2006. PMID: 16413387
-
Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter.Am J Respir Cell Mol Biol. 2003 Jun;28(6):648-54. doi: 10.1165/rcmb.2002-0095OC. Am J Respir Cell Mol Biol. 2003. PMID: 12760962
-
Cell differentiation dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt signaling resulting in proliferation and migration of colorectal cancer cells.J Cell Biochem. 2006 Mar 1;97(4):709-23. doi: 10.1002/jcb.20672. J Cell Biochem. 2006. PMID: 16215992
-
The CC chemokine CCL20 and its receptor CCR6.Cytokine Growth Factor Rev. 2003 Oct;14(5):409-26. doi: 10.1016/s1359-6101(03)00049-2. Cytokine Growth Factor Rev. 2003. PMID: 12948524 Review.
-
[Study on migration property of mesenchymal stem cells-review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009 Aug;17(4):1101-5. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009. PMID: 19698270 Review. Chinese.
Cited by
-
Development and application of oncolytic viruses as the nemesis of tumor cells.Front Microbiol. 2023 Jun 12;14:1188526. doi: 10.3389/fmicb.2023.1188526. eCollection 2023. Front Microbiol. 2023. PMID: 37440883 Free PMC article. Review.
-
Mesenchymal stem cell-derived exosomes as new tools for delivery of miRNAs in the treatment of cancer.Front Bioeng Biotechnol. 2022 Sep 26;10:956563. doi: 10.3389/fbioe.2022.956563. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36225602 Free PMC article. Review.
-
Application of Mesenchymal Stem Cells for Therapeutic Agent Delivery in Anti-tumor Treatment.Front Pharmacol. 2018 Mar 20;9:259. doi: 10.3389/fphar.2018.00259. eCollection 2018. Front Pharmacol. 2018. PMID: 29615915 Free PMC article. Review.
-
Multipotent mesenchymal stromal cells enhance insulin secretion from human islets via N-cadherin interaction and prolong function of transplanted encapsulated islets in mice.Stem Cell Res Ther. 2017 Sep 29;8(1):199. doi: 10.1186/s13287-017-0646-7. Stem Cell Res Ther. 2017. PMID: 28962589 Free PMC article.
-
IL-8, GRO and MCP-1 produced by hepatocellular carcinoma microenvironment determine the migratory capacity of human bone marrow-derived mesenchymal stromal cells without affecting tumor aggressiveness.Oncotarget. 2016 Jun 25;8(46):80235-80248. doi: 10.18632/oncotarget.10288. eCollection 2017 Oct 6. Oncotarget. 2016. PMID: 29113298 Free PMC article.
References
-
- Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA. and Ruadkow IA. (1974). Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 2:83–92 - PubMed
-
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S. and Gianni AM. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843 - PubMed
-
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E. and Dazzi F. (2003). Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101:3722–3729 - PubMed
-
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V. and Uccelli A. (2006). Human mesenchymal stem cells modulate B-cell functions. Blood 107:367–372 - PubMed
-
- Glennie S, Soeiro I, Dyson PJ, Lam EW. and Dazzi F. (2005). Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105:2821–2827 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

