Iron complexation to histone deacetylase inhibitors SAHA and LAQ824 in PEGylated liposomes can considerably improve pharmacokinetics in rats
- PMID: 25579435
- PMCID: PMC4560202
- DOI: 10.18433/j3ts4v
Iron complexation to histone deacetylase inhibitors SAHA and LAQ824 in PEGylated liposomes can considerably improve pharmacokinetics in rats
Abstract
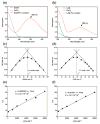
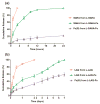
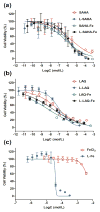
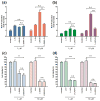
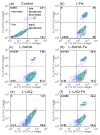
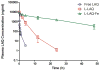
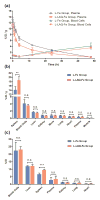
The formulation of histone deacetylase inhibitors (HDACi) is challenging due to poor water solubility and rapid elimination of drugs in vivo. This study investigated the effects of complexing iron (Fe3+) to the HDACi suberoylanilide hydroxamic acid (SAHA) and LAQ824 (LAQ) prior to their encapsulation into PEGylated liposomes, and investigated whether this technique could improve drug solubility, in vitro release and in vivo pharmacokinetic (PK) properties. METHODS. The reaction stoichiometry, binding constants and solubility were measured for Fe complexes of SAHA and LAQ. The complexes were passively encapsulated into PEGylated liposomes and characterized by size distribution, zeta-potential, encapsulation efficiency (EE), and in vitro drug release studies. PC-3 cells were used to verify the in vitro anticancer activity of the formulations. In vivo pharmacokinetic properties of liposomal LAQ-Fe (L-LAQ-Fe) was evaluated in rats. RESULTS. SAHA and LAQ form complexes with Fe at 1:1 stoichiometric ratio, with a binding constant on the order of 104 M-1. Fe complexation improved the aqueous solubility and the liposomal encapsulation efficiency of SAHA and LAQ (29-35% EE, final drug concentration > 1 mM). Liposomal encapsulated complexes (L-HDACi-Fe) exhibited sustained in vitro release properties compared to L-HDACi but cytotoxicity on PC-3 cells was comparable to free drugs. The PK of L-LAQ-Fe revealed 15-fold improvement in the plasma t1/2 (12.11 h)and 211-fold improvement in the AUC∞ (105.7 µg·h/ml) compared to free LAQ (0.79 h, 0.5 µg·h/ml). Similarly, the plasma t1/2 of Fe was determined to be 11.83 h in a separate experiment using radioactive Fe-59. The majority of Fe-59 activity was found in liver and spleen of rats and correlates with liposomal uptake by the mononuclear phagocyte system. CONCLUSIONS. We have demonstrated that encapsulation of Fe complexes of HDACi into PEGylated liposomes can improve overall drug aqueous solubility, in vitro release and in vivo pharmacokinetic properties.
Conflict of interest statement
The authors report no conflict of interest.
Figures
Similar articles
-
Antitumor effect of liposomal histone deacetylase inhibitor-lipid conjugates in vitro.Chem Pharm Bull (Tokyo). 2011;59(11):1386-92. doi: 10.1248/cpb.59.1386. Chem Pharm Bull (Tokyo). 2011. PMID: 22041075
-
Enhanced pharmacodynamic and antitumor properties of a histone deacetylase inhibitor encapsulated in liposomes or ErbB2-targeted immunoliposomes.Clin Cancer Res. 2005 May 1;11(9):3392-401. doi: 10.1158/1078-0432.CCR-04-2445. Clin Cancer Res. 2005. PMID: 15867240
-
Preparation, Characterization, and In Vitro Pharmacodynamics and Pharmacokinetics Evaluation of PEGylated Urolithin A Liposomes.AAPS PharmSciTech. 2021 Jan 6;22(1):26. doi: 10.1208/s12249-020-01890-y. AAPS PharmSciTech. 2021. PMID: 33404864
-
Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies.Clin Pharmacokinet. 2003;42(5):419-36. doi: 10.2165/00003088-200342050-00002. Clin Pharmacokinet. 2003. PMID: 12739982 Review.
-
Immunomodulatory effects of histone deacetylase inhibitors.Curr Mol Med. 2013 May;13(4):640-7. doi: 10.2174/1566524011313040013. Curr Mol Med. 2013. PMID: 23061676 Review.
Cited by
-
β-Cyclodextrin-poly (β-Amino Ester) Nanoparticles Are a Generalizable Strategy for High Loading and Sustained Release of HDAC Inhibitors.ACS Appl Mater Interfaces. 2021 May 12;13(18):20960-20973. doi: 10.1021/acsami.0c22587. Epub 2021 Apr 27. ACS Appl Mater Interfaces. 2021. PMID: 33905245 Free PMC article.
-
Autologous Red Blood Cell Delivery of Betamethasone Phosphate Sodium for Long Anti-Inflammation.Pharmaceutics. 2018 Dec 18;10(4):286. doi: 10.3390/pharmaceutics10040286. Pharmaceutics. 2018. PMID: 30567356 Free PMC article.
-
Epigenetics in Breast Cancer Therapy-New Strategies and Future Nanomedicine Perspectives.Cancers (Basel). 2020 Dec 3;12(12):3622. doi: 10.3390/cancers12123622. Cancers (Basel). 2020. PMID: 33287297 Free PMC article. Review.
-
Epigenetic drugs in cancer therapy.Cancer Metastasis Rev. 2025 Feb 26;44(1):37. doi: 10.1007/s10555-025-10253-7. Cancer Metastasis Rev. 2025. PMID: 40011240 Free PMC article. Review.
-
HDAC inhibitors: Targets for tumor therapy, immune modulation and lung diseases.Transl Oncol. 2022 Feb;16:101312. doi: 10.1016/j.tranon.2021.101312. Epub 2021 Dec 16. Transl Oncol. 2022. PMID: 34922087 Free PMC article. Review.
References
-
- Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nature reviews Drug discovery. 2002;1(4):287–99. - PubMed
-
- Carew JS, Giles FJ, Nawrocki ST. Histone deacetylase inhibitors: mechanisms of cell death and promise in combination cancer therapy. Cancer letters. 2008;269(1):7–17. - PubMed
-
- Inoue T, Hiratsuka M, Osaki M, Oshimura M. The molecular biology of mammalian SIRT proteins: SIRT2 in cell cycle regulation. Cell Cycle. 2007;6(9):1011–8. - PubMed
-
- Pan LN, Lu J, Huang B. HDAC inhibitors: a potential new category of anti-tumor agents. Cellular & molecular immunology. 2007;4(5):337–43. - PubMed
-
- Cote S, Rosenauer A, Bianchini A, Seiter K, Vandewiele J, Nervi C, et al. Response to histone deacetylase inhibition of novel PML/RARalpha mutants detected in retinoic acid-resistant APL cells. Blood. 2002;100(7):2586–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

