Proinsulin misfolding and endoplasmic reticulum stress during the development and progression of diabetes
- PMID: 25579745
- PMCID: PMC4404191
- DOI: 10.1016/j.mam.2015.01.001
Proinsulin misfolding and endoplasmic reticulum stress during the development and progression of diabetes
Abstract
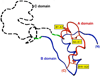
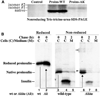
To maintain copious insulin granule stores in the face of ongoing metabolic demand, pancreatic beta cells must produce large quantities of proinsulin, the insulin precursor. Proinsulin biosynthesis can account for up to 30-50% of total cellular protein synthesis of beta cells. This puts pressure on the beta cell secretory pathway, especially the endoplasmic reticulum (ER), where proinsulin undergoes its initial folding, including the formation of three evolutionarily conserved disulfide bonds. In normal beta cells, up to 20% of newly synthesized proinsulin may fail to reach its native conformation, suggesting that proinsulin is a misfolding-prone protein. Misfolded proinsulin molecules can either be refolded to their native structure or degraded through ER associated degradation (ERAD) and autophagy. These degraded molecules decrease proinsulin yield but do not otherwise compromise beta cell function. However, under certain pathological conditions, proinsulin misfolding increases, exceeding the genetically determined threshold of beta cells to handle the misfolded protein load. This results in accumulation of misfolded proinsulin in the ER - a causal factor leading to beta cell failure and diabetes. In patients with Mutant INS-gene induced diabetes of Youth (MIDY), increased proinsulin misfolding due to insulin gene mutations is the primary defect operating as a "first hit" to beta cells. Additionally, increased proinsulin misfolding can be secondary to an unfavorable ER folding environment due to genetic and/or environmental factors. Under these conditions, increased wild-type proinsulin misfolding becomes a "second hit" to the ER and beta cells, aggravating beta cell failure and diabetes. In this article, we describe our current understanding of the normal proinsulin folding pathway in the ER, and then review existing links between proinsulin misfolding, ER dysfunction, and beta cell failure in the development and progression of type 2, type 1, and some monogenic forms of diabetes.
Keywords: Beta cell; Diabetes; Endoplasmic reticulum stress; Insulin biosynthesis; Proinsulin folding and misfolding.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures



References
-
- Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, Mori K. ATF6 Is a Transcription Factor Specializing in the Regulation of Quality Control Proteins in the Endoplasmic Reticulum. Cell Structure and Function. 2008;33(1):75–89. - PubMed
-
- Alarcón C, Lincoln B, Rhodes CJ. The biosynthesis of the subtilisin-related proprotein convertase PC3, but no that of the PC2 convertase, is regulated by glucose in parallel to proinsulin biosynthesis in rat pancreatic islets. Journal of Biological Chemistry. 1993;268(6):4276–4280. - PubMed
-
- Appenzeller-Herzog C. Glutathione- and non-glutathione-based oxidant control in the endoplasmic reticulum. Journal of Cell Science. 2011;124(6):847–855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

