Immunogenetics of type 1 diabetes mellitus
- PMID: 25579746
- PMCID: PMC4548800
- DOI: 10.1016/j.mam.2014.12.004
Immunogenetics of type 1 diabetes mellitus
Abstract
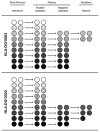
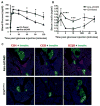
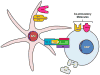
Type 1 diabetes mellitus (T1DM) is an autoimmune disease arising through a complex interaction of both genetic and immunologic factors. Similar to the majority of autoimmune diseases, T1DM usually has a relapsing remitting disease course with autoantibody and T cellular responses to islet autoantigens, which precede the clinical onset of the disease process. The immunological diagnosis of autoimmune diseases relies primarily on the detection of autoantibodies in the serum of T1DM patients. Although their pathogenic significance remains uncertain, they have the practical advantage of serving as surrogate biomarkers for predicting the clinical onset of T1DM. Type 1 diabetes is a polygenic disease with a small number of genes having large effects (i.e. HLA), and a large number of genes having small effects. Risk of T1DM progression is conferred by specific HLA DR/DQ alleles [e.g., DRB1*03-DQB1*0201 (DR3) or DRB1*04-DQB1*0302 (DR4)]. In addition, HLA alleles such as DQB1*0602 are associated with dominant protection from T1DM in multiple populations. A discordance rate of greater than 50% between monozygotic twins indicates a potential involvement of environmental factors on disease development. Viral infections may play a role in the chain of events leading to disease, albeit conclusive evidence linking infections with T1DM remains to be firmly established. Two syndromes have been described in which an immune-mediated form of diabetes occurs as the result of a single gene defect. These syndromes are termed autoimmune polyglandular syndrome type I (APS-I) or autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), and X-linked poyendocrinopathy, immune dysfunction and diarrhea (XPID). These two syndromes are unique models to understand the mechanisms involved in the loss of tolerance to self-antigens in autoimmune diabetes and its associated organ-specific autoimmune disorders. A growing number of animal models of these diseases have greatly helped elucidate the immunologic mechanisms leading to autoimmune diabetes.
Keywords: Genetics; Immunology; Pathogenesis; Type 1 diabetes.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


References
-
- Achenbach P, Warncke K, Reiter J, Naserke HE, Williams AJ, Bingley PJ, Bonifacio E, Ziegler AG. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes. 2004;53(2):384–392. - PubMed
-
- Allen C, Palta M, D’Alessio DJ. Incidence and differences in urban-rural seasonal variation of type 1 (insulin-dependent) diabetes in Wisconsin. Diabetologia. 1986;29:629–633. - PubMed
-
- Allen JS, Pang K, Skowera A, Ellis R, Rackham C, Lozanoska-Ochser B, Tree T, Leslie RD, Tremble JM, Dayan CM, Peakman M. Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes. 2009;58(1):138–145. - PMC - PubMed
-
- Alkanani AK, Hara N, Gianani R, Zipris D. Kilham rat virus-induced type 1 diabetes involves beta cell infection and intra-islet JAK-STAT activation prior to insulitis. Virology. 2014;468–470C:19–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

