Role of bacteriophages in STEC infections: new implications for the design of prophylactic and treatment approaches
- PMID: 25580222
- PMCID: PMC4288416
- DOI: 10.12688/f1000research.3718.2
Role of bacteriophages in STEC infections: new implications for the design of prophylactic and treatment approaches
Abstract
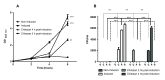
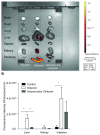
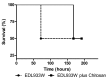
Shiga toxin (Stx) is considered the main virulence factor in Shiga toxin-producing Escherichia coli (STEC) infections. Previously we reported the expression of biologically active Stx by eukaryotic cells in vitro and in vivo following transfection with plasmids encoding Stx under control of the native bacterial promoter (1,2). Since stx genes are present in the genome of lysogenic bacteriophages, here we evaluated the relevance of bacteriophages during STEC infection. We used the non-pathogenic E. coli C600 strain carrying a lysogenic 933W mutant bacteriophage in which the stx operon was replaced by a gene encoding the green fluorescent protein (GFP). Tracking GFP expression using an In Vivo Imaging System (IVIS), we detected fluorescence in liver, kidney, and intestine of mice infected with the recombinant E. coli strain after treatment with ciprofloxacin, which induces the lytic replication and release of bacteriophages. In addition, we showed that chitosan, a linear polysaccharide composed of d-glucosamine residues and with a number of commercial and biomedical uses, had strong anti-bacteriophage effects, as demonstrated at in vitro and in vivo conditions. These findings bring promising perspectives for the prevention and treatment of haemolytic uremic syndrome (HUS) cases.
Conflict of interest statement
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

