Adeno-associated viral (AAV) vectors do not efficiently target muscle satellite cells
- PMID: 25580445
- PMCID: PMC4288464
- DOI: 10.1038/mtm.2014.38
Adeno-associated viral (AAV) vectors do not efficiently target muscle satellite cells
Abstract
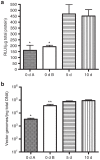
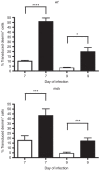
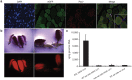
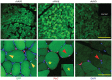
Adeno-associated viral (AAV) vectors are becoming an important tool for gene therapy of numerous genetic and other disorders. Several recombinant AAV vectors (rAAV) have the ability to transduce striated muscles in a variety of animals following intramuscular and intravascular administration, and have attracted widespread interest for therapy of muscle disorders such as the muscular dystrophies. However, most studies have focused on the ability to transduce mature muscle cells, and have not examined the ability to target myogenic stem cells such as skeletal muscle satellite cells. Here we examined the relative ability of rAAV vectors derived from AAV6 to target myoblasts, myocytes and myotubes in culture and satellite cells and myofibers in vivo. AAV vectors are able to transduce proliferating myoblasts in culture, albeit with reduced efficiency relative to post-mitotic myocytes and myotubes. In contrast, quiescent satellite cells are refractory to transduction in adult mice. These results suggest that while muscle disorders characterized by myofiber regeneration can be slowed or halted by AAV transduction, little if any vector transduction can be obtained in myogenic stems cells that might other wise support ongoing muscle regeneration.
Figures




References
-
- Smith RH. Adeno-associated virus integration: virus versus vector. Gene Ther. 2008;15:817–822. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

