Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth
- PMID: 25580734
- PMCID: PMC4312504
- DOI: 10.1038/ncb3090
Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth
Abstract
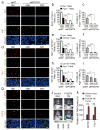
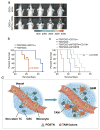
Tumour-associated macrophages (TAMs) are enriched in glioblastoma multiformes (GBMs) that contain glioma stem cells (GSCs) at the apex of their cellular hierarchy. The correlation between TAM density and glioma grade suggests a supportive role for TAMs in tumour progression. Here we interrogated the molecular link between GSCs and TAM recruitment in GBMs and demonstrated that GSCs secrete periostin (POSTN) to recruit TAMs. TAM density correlates with POSTN levels in human GBMs. Silencing POSTN in GSCs markedly reduced TAM density, inhibited tumour growth, and increased survival of mice bearing GSC-derived xenografts. We found that TAMs in GBMs are not brain-resident microglia, but mainly monocyte-derived macrophages from peripheral blood. Disrupting POSTN specifically attenuated the tumour-supportive M2 type of TAMs in xenografts. POSTN recruits TAMs through the integrin αvβ₃ as blocking this signalling by an RGD peptide inhibited TAM recruitment. Our findings highlight the possibility of improving GBM treatment by targeting POSTN-mediated TAM recruitment.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






Comment in
-
A niche role for periostin and macrophages in glioblastoma.Nat Cell Biol. 2015 Feb;17(2):107-9. doi: 10.1038/ncb3095. Nat Cell Biol. 2015. PMID: 25633271
-
CNS cancer: periostin-a new potential target for the treatment of glioblastoma.Nat Rev Clin Oncol. 2015 Mar;12(3):128. doi: 10.1038/nrclinonc.2015.15. Epub 2015 Feb 3. Nat Rev Clin Oncol. 2015. PMID: 25645602 No abstract available.
-
Hostile takeover: glioma stem cells recruit TAMs to support tumor progression.Cell Stem Cell. 2015 Mar 5;16(3):219-20. doi: 10.1016/j.stem.2015.02.008. Cell Stem Cell. 2015. PMID: 25748928
References
-
- Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20:E1. - PubMed
-
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. - PubMed
-
- Hao C, et al. Cytokine and cytokine receptor mRNA expression in human glioblastomas: evidence of Th1, Th2 and Th3 cytokine dysregulation. Acta Neuropathol. 2002;103:171–178. - PubMed
-
- Morford LA, Dix AR, Brooks WH, Roszman TL. Apoptotic elimination of peripheral T lymphocytes in patients with primary intracranial tumors. J Neurosurg. 1999;91:935–946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

