Human iPSC-derived motoneurons harbouring TARDBP or C9ORF72 ALS mutations are dysfunctional despite maintaining viability
- PMID: 25580746
- PMCID: PMC4338554
- DOI: 10.1038/ncomms6999
Human iPSC-derived motoneurons harbouring TARDBP or C9ORF72 ALS mutations are dysfunctional despite maintaining viability
Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease for which a greater understanding of early disease mechanisms is needed to reveal novel therapeutic targets. We report the use of human induced pluripotent stem cell (iPSC)-derived motoneurons (MNs) to study the pathophysiology of ALS. We demonstrate that MNs derived from iPSCs obtained from healthy individuals or patients harbouring TARDBP or C9ORF72 ALS-causing mutations are able to develop appropriate physiological properties. However, patient iPSC-derived MNs, independent of genotype, display an initial hyperexcitability followed by progressive loss of action potential output and synaptic activity. This loss of functional output reflects a progressive decrease in voltage-activated Na(+) and K(+) currents, which occurs in the absence of overt changes in cell viability. These data implicate early dysfunction or loss of ion channels as a convergent point that may contribute to the initiation of downstream degenerative pathways that ultimately lead to MN loss in ALS.
Figures
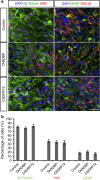
 ; *P<0.05; factorial ANOVA).
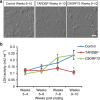
; *P<0.05; factorial ANOVA).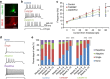
 with lines of best fit; *significantly different to control, P<0.05; ***significantly different to control, P<0.0001; linear model with multiple contrast for the gradient values, and adjusted with Bonferroni correction). (d) Examples of the four categories of firing observed in iPSC-derived MNs (repetitive, adaptive, single or no firing). (e) Proportion of cells exhibiting each firing category in iPSC-derived MNs from control (n=702), TARDBP (n=380) and C9ORF72 (n=239) lines across weeks 3–10 post plating (***significantly different to control, P<0.0001; logistic regression with multiple Wald’s test and Bonferroni correction).
with lines of best fit; *significantly different to control, P<0.05; ***significantly different to control, P<0.0001; linear model with multiple contrast for the gradient values, and adjusted with Bonferroni correction). (d) Examples of the four categories of firing observed in iPSC-derived MNs (repetitive, adaptive, single or no firing). (e) Proportion of cells exhibiting each firing category in iPSC-derived MNs from control (n=702), TARDBP (n=380) and C9ORF72 (n=239) lines across weeks 3–10 post plating (***significantly different to control, P<0.0001; logistic regression with multiple Wald’s test and Bonferroni correction).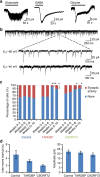

 ; *significantly different to controls, P<0.05; ***significantly different to controls, P<0.0001; #significant difference between patient lines, P<0.05; ##significant difference between patient lines, P<0.001; linear model with multiple Wald’s tests and Bonferroni correction).
; *significantly different to controls, P<0.05; ***significantly different to controls, P<0.0001; #significant difference between patient lines, P<0.05; ##significant difference between patient lines, P<0.001; linear model with multiple Wald’s tests and Bonferroni correction).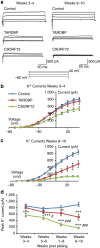
 ; *significantly different to control, P<0.05; ***significantly different to control, P<0.0001; #significant difference between patient lines, P<0.05; ###significant difference between patient lines, P<0.0001; linear model with multiple Wald’s tests and Bonferroni correction).
; *significantly different to control, P<0.05; ***significantly different to control, P<0.0001; #significant difference between patient lines, P<0.05; ###significant difference between patient lines, P<0.0001; linear model with multiple Wald’s tests and Bonferroni correction).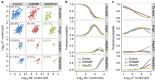
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

