Quantitative EEG is an objective, sensitive, and reliable indicator of transient anesthetic effects during Wada tests
- PMID: 25580802
- PMCID: PMC4385440
- DOI: 10.1097/WNP.0000000000000154
Quantitative EEG is an objective, sensitive, and reliable indicator of transient anesthetic effects during Wada tests
Abstract
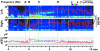
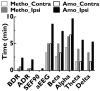
The intracarotid amobarbital or Wada procedure is a component of the presurgical evaluation for refractory epilepsy, during which monitoring the onset and offset of transient anesthetic effects is critical. In this study, the authors characterized changes of 8 quantitative measures during 26 Wada tests, which included alpha, beta, theta, and delta powers, alpha/delta power ratio, beta/delta power ratio, median amplitude-integrated EEG, and 90% spectral edge frequency (SEF90), and correlated them with contralateral hemiplegia. The authors found that on the side of injection, delta and theta powers, alpha/delta power ratio, beta/delta power ratio, and SEF90 peaked within 1 minute after injection of 70 to 150 mg amobarbital or 4 to 7 mg methohexital. When contralateral arm strength returned to 3/5, delta power and amplitude-integrated EEG decayed on average 24% and 19%, respectively, for amobarbital, similar to that of methohexital (27% and 18%). Because delta power resolution most closely mirrored that of the hemiplegia and amplitude-integrated EEG had the highest signal/noise ratio, these quantitative values appear to be the best measures for decay of anesthetic effects. Increase in alpha power persisted longest, and therefore may be the best measure of late residual anesthetic effects.
Figures




References
-
- Acharya JN, Dinner DS. Use of the intracarotid amobarbital procedure in the evaluation of memory. J Clin Neurophysiol. 1997;14:311–325. - PubMed
-
- Ahern GL, Labiner DM, Talwar D, et al. Quantitative analysis of the electroencephalogram in the intracarotid amobarbital procedure: Ii. Coherence analysis. Journal of clinical neurophysiology: official publication of the American Electroencephalographic Society. 1995;12:285–290. - PubMed
-
- Akman CI, Micic V, Thompson A, et al. Seizure detection using digital trend analysis: Factors affecting utility. Epilepsy Res. 2011;93:66–72. - PubMed

