Lipid peroxidation is essential for α-synuclein-induced cell death
- PMID: 25580849
- PMCID: PMC4471127
- DOI: 10.1111/jnc.13024
Lipid peroxidation is essential for α-synuclein-induced cell death
Abstract
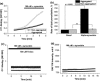
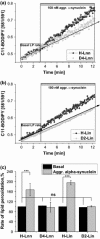
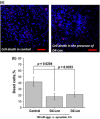
Parkinson's disease is the second most common neurodegenerative disease and its pathogenesis is closely associated with oxidative stress. Deposition of aggregated α-synuclein (α-Syn) occurs in familial and sporadic forms of Parkinson's disease. Here, we studied the effect of oligomeric α-Syn on one of the major markers of oxidative stress, lipid peroxidation, in primary co-cultures of neurons and astrocytes. We found that oligomeric but not monomeric α-Syn significantly increases the rate of production of reactive oxygen species, subsequently inducing lipid peroxidation in both neurons and astrocytes. Pre-incubation of cells with isotope-reinforced polyunsaturated fatty acids (D-PUFAs) completely prevented the effect of oligomeric α-Syn on lipid peroxidation. Inhibition of lipid peroxidation with D-PUFAs further protected cells from cell death induced by oligomeric α-Syn. Thus, lipid peroxidation induced by misfolding of α-Syn may play an important role in the cellular mechanism of neuronal cell loss in Parkinson's disease. We have found that aggregated α-synuclein-induced production of reactive oxygen species (ROS) that subsequently stimulates lipid peroxidation and cell death in neurons and astrocytes. Specific inhibition of lipid peroxidation by incubation with reinforced polyunsaturated fatty acids (D-PUFAs) completely prevented the effect of α-synuclein on lipid peroxidation and cell death.
Keywords: deuterated PUFA; lipid peroxidation; oxidative stress; α-synuclein.
© 2015 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of The International Society for Neurochemistry.
Figures



References
-
- Alessandri J. M., Guesnet P., Vancassel S., Astorg P., Denis I., Langelier B., Aid S., Poumes‐Ballihaut C., Champeil‐Potokar G. and Lavialle M. (2004) Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod. Nutr. Dev. 44, 509–538. - PubMed
-
- Amer D. A., Irvine G. B. and El‐Agnaf O. M. (2006) Inhibitors of alpha‐synuclein oligomerization and toxicity: a future therapeutic strategy for Parkinson's disease and related disorders. Exp. Brain Res. 173, 223–233. - PubMed
-
- Brenna J. T. and Carlson S. E. (2014) Docosahexaenoic acid and human brain development: evidence that a dietary supply is needed for optimal development. J. Hum. Evol. 77, 99–106. - PubMed
-
- Brenner R. R. (1974) The oxidative desaturation of unsaturated fatty acids in animals. Mol. Cell. Biochem. 3, 41–52. - PubMed
-
- Broersen K., van den Brink D., Fraser G., Goedert M. and Davletov B. (2006) Alpha‐synuclein adopts an alpha‐helical conformation in the presence of polyunsaturated fatty acids to hinder micelle formation. Biochemistry 45, 15610–15616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

