Members of the chloride intracellular ion channel protein family demonstrate glutaredoxin-like enzymatic activity
- PMID: 25581026
- PMCID: PMC4291220
- DOI: 10.1371/journal.pone.0115699
Members of the chloride intracellular ion channel protein family demonstrate glutaredoxin-like enzymatic activity
Abstract
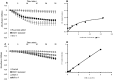
The Chloride Intracellular Ion Channel (CLIC) family consists of six evolutionarily conserved proteins in humans. Members of this family are unusual, existing as both monomeric soluble proteins and as integral membrane proteins where they function as chloride selective ion channels, however no function has previously been assigned to their soluble form. Structural studies have shown that in the soluble form, CLIC proteins adopt a glutathione S-transferase (GST) fold, however, they have an active site with a conserved glutaredoxin monothiol motif, similar to the omega class GSTs. We demonstrate that CLIC proteins have glutaredoxin-like glutathione-dependent oxidoreductase enzymatic activity. CLICs 1, 2 and 4 demonstrate typical glutaredoxin-like activity using 2-hydroxyethyl disulfide as a substrate. Mutagenesis experiments identify cysteine 24 as the catalytic cysteine residue in CLIC1, which is consistent with its structure. CLIC1 was shown to reduce sodium selenite and dehydroascorbate in a glutathione-dependent manner. Previous electrophysiological studies have shown that the drugs IAA-94 and A9C specifically block CLIC channel activity. These same compounds inhibit CLIC1 oxidoreductase activity. This work for the first time assigns a functional activity to the soluble form of the CLIC proteins. Our results demonstrate that the soluble form of the CLIC proteins has an enzymatic activity that is distinct from the channel activity of their integral membrane form. This CLIC enzymatic activity may be important for protecting the intracellular environment against oxidation. It is also likely that this enzymatic activity regulates the CLIC ion channel function.
Conflict of interest statement
Figures






References
-
- Valenzuela SM, Martin DK, Por SB, Robbins JM, Warton K, et al. (1997) Molecular cloning and expression of a chloride ion channel of cell nuclei. Journal of Biological Chemistry 272:12575–12582. - PubMed
-
- Heiss NS, Poustka A (1997) Genomic structure of a novel chloride channel gene, CLIC2, in Xq28. Genomics 45:224–228. - PubMed
-
- Qian Z, Okuhara D, Abe MK, Rosner MR (1999) Molecular cloning and characterization of a mitogen-activated protein kinase-associated intracellular chloride channel. Journal of Biological Chemistry 274:1621–1627. - PubMed
-
- Duncan RR, Westwood PK, Boyd A, Ashley RH (1997) Rat brain p64H1, expression of a new member of the p64 chloride channel protein family in endoplasmic reticulum. Journal of Biological Chemistry 272:23880–23886. - PubMed
-
- Howell S, Duncan RR, Ashley RH (1996) Identification and characterisation of a homologue of p64 in rat tissues. FEBS Letters 390:207–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

