Kruppel-like factor KLF4 facilitates cutaneous wound healing by promoting fibrocyte generation from myeloid-derived suppressor cells
- PMID: 25581502
- PMCID: PMC4402119
- DOI: 10.1038/jid.2015.3
Kruppel-like factor KLF4 facilitates cutaneous wound healing by promoting fibrocyte generation from myeloid-derived suppressor cells
Abstract
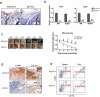
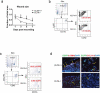
Pressure ulcers (PUs) are serious skin injuries whereby the wound healing process is frequently stalled in the inflammatory phase. Myeloid-derived suppressor cells (MDSCs) accumulate as a result of inflammation and promote cutaneous wound healing by mechanisms that are not fully understood. Recently, MDSCs have been shown to differentiate into fibrocytes, which serve as emerging effector cells that enhance cell proliferation in wound healing. We postulate that in wound healing MDSCs not only execute their immunosuppressive function to regulate inflammation but also stimulate cell proliferation once they differentiate into fibrocytes. In the current study, by using full-thickness and PU mouse models, we found that Kruppel-like factor 4 (KLF4) deficiency resulted in decreased accumulation of MDSCs and fibrocytes, and wound healing was significantly delayed. Conversely, KLF4 activation by the plant-derived product Mexicanin I increased the number of MDSCs and fibrocytes and accelerated the wound healing. Collectively, our study revealed a previously unreported function of MDSCs in cutaneous wound healing and identified Mexicanin I as a potential agent to accelerate PU wound healing.
Figures




References
-
- Ansel KM, Djuretic I, Tanasa B, et al. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–56. - PubMed
-
- Black JM, Edsberg LE, Baharestani MM, et al. Pressure ulcers: avoidable or unavoidable? Results of the National Pressure Ulcer Advisory Panel Consensus Conference. Ostomy Wound Manage. 2011;57:24–37. - PubMed
-
- Bujnicki T, Wilczek C, Schomburg C, et al. Inhibition of Myb-dependent gene expression by the sesquiterpene lactone mexicanin-I. Leukemia. 2012;26:615–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

