Multi-center reproducibility of neurochemical profiles in the human brain at 7 T
- PMID: 25581510
- PMCID: PMC4339538
- DOI: 10.1002/nbm.3252
Multi-center reproducibility of neurochemical profiles in the human brain at 7 T
Abstract
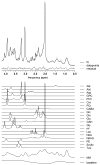
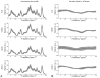
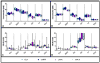
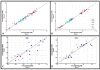
The purpose of this work was to harmonize data acquisition and post-processing of single voxel proton MRS ((1) H-MRS) at 7 T, and to determine metabolite concentrations and the accuracy and reproducibility of metabolite levels in the adult human brain. This study was performed in compliance with local institutional human ethics committees. The same seven subjects were each examined twice using four different 7 T MR systems from two different vendors using an identical semi-localization by adiabatic selective refocusing spectroscopy sequence. Neurochemical profiles were obtained from the posterior cingulate cortex (gray matter, GM) and the corona radiata (white matter, WM). Spectra were analyzed with LCModel, and sources of variation in concentrations ('subject', 'institute' and 'random') were identified with a variance component analysis. Concentrations of 10-11 metabolites, which were corrected for T1 , T2 , magnetization transfer effects and partial volume effects, were obtained with mean Cramér-Rao lower bounds below 20%. Data variances and mean concentrations in GM and WM were comparable for all institutions. The primary source of variance for glutamate, myo-inositol, scyllo-inositol, total creatine and total choline was between subjects. Variance sources for all other metabolites were associated with within-subject and system noise, except for total N-acetylaspartate, glutamine and glutathione, which were related to differences in signal-to-noise ratio and in shimming performance between vendors. After multi-center harmonization of acquisition and post-processing protocols, metabolite concentrations and the sizes and sources of their variations were established for neurochemical profiles in the healthy brain at 7 T, which can be used as guidance in future studies quantifying metabolite and neurotransmitter concentrations with (1) H-MRS at ultra-high magnetic field.
Keywords: 7 T; MRS; neurochemical profiling; reproducibility; semi-LASER; test-retest; ultra-high field strength.
Copyright © 2015 John Wiley & Sons, Ltd.
Figures


References
-
- Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, Bolan PJ, Brindle KM, Cudalbu C, Dincer A, Dydak U, Emir UE, Frahm J, Gonzalez RG, Gruber S, Gruetter R, Gupta RK, Heerschap A, Henning A, Hetherington HP, Howe FA, Huppi PS, Hurd RE, Kantarci K, Klomp DW, Kreis R, Kruiskamp MJ, Leach MO, Lin AP, Luijten PR, Marjanska M, Maudsley AA, Meyerhoff DJ, Mountford CE, Nelson SJ, Pamir MN, Pan JW, Peet AC, Poptani H, Posse S, Pouwels PJ, Ratai EM, Ross BD, Scheenen TW, Schuster C, Smith IC, Soher BJ, Tkac I, Vigneron DB, Kauppinen RA. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270(3):658–679. - PMC - PubMed
-
- Duarte JM, Lei H, Mlynarik V, Gruetter R. The neurochemical profile quantified by in vivo 1H NMR spectroscopy. Neuroimage. 2012;61(2):342–362. - PubMed
-
- Ugurbil K, Adriany G, Andersen P, Chen W, Garwood M, Gruetter R, Henry PG, Kim SG, Lieu H, Tkac I, Vaughan T, Van De Moortele PF, Yacoub E, Zhu XH. Ultrahigh field magnetic resonance imaging and spectroscopy. Magnetic Resonance Imaging. 2003;21(10):1263–1281. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

