Autologous bone marrow mononuclear cells reduce therapeutic intensity for severe traumatic brain injury in children
- PMID: 25581630
- PMCID: PMC4351120
- DOI: 10.1097/PCC.0000000000000324
Autologous bone marrow mononuclear cells reduce therapeutic intensity for severe traumatic brain injury in children
Abstract
Objectives: The devastating effect of traumatic brain injury is exacerbated by an acute secondary neuroinflammatory response, clinically manifest as elevated intracranial pressure due to cerebral edema. The treatment effect of cell-based therapies in the acute post-traumatic brain injury period has not been clinically studied although preclinical data demonstrate that bone marrow-derived mononuclear cell infusion down-regulates the inflammatory response. Our study evaluates whether pediatric traumatic brain injury patients receiving IV autologous bone marrow-derived mononuclear cells within 48 hours of injury experienced a reduction in therapeutic intensity directed toward managing elevated intracranial pressure relative to matched controls.
Design: The study was a retrospective cohort design comparing pediatric patients in a phase I clinical trial treated with IV autologous bone marrow-derived mononuclear cells (n = 10) to a control group of age- and severity-matched children (n = 19).
Setting: The study setting was at Children's Memorial Hermann Hospital, an American College of Surgeons Level 1 Pediatric Trauma Center and teaching hospital for the University of Texas Health Science Center at Houston from 2000 to 2008.
Patients: Study patients were 5-14 years with postresuscitation Glasgow Coma Scale scores of 5-8.
Interventions: The treatment group received 6 million autologous bone marrow-derived mononuclear cells/kg body weight IV within 48 hours of injury. The control group was treated in an identical fashion, per standard of care, guided by our traumatic brain injury management protocol, derived from American Association of Neurological Surgeons guidelines.
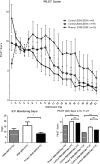
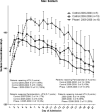
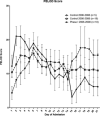
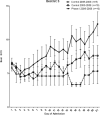
Measurements and main results: The primary measure was the Pediatric Intensity Level of Therapy scale used to quantify treatment of elevated intracranial pressure. Secondary measures included the Pediatric Logistic Organ Dysfunction score and days of intracranial pressure monitoring as a surrogate for length of neurointensive care. A repeated-measure mixed model with marginal linear predictions identified a significant reduction in the Pediatric Intensity Level of Therapy score beginning at 24 hours posttreatment through week 1 (p < 0.05). This divergence was also reflected in the Pediatric Logistic Organ Dysfunction score following the first week. The duration of intracranial pressure monitoring was 8.2 ± 1.3 days in the treated group and 15.6 ± 3.5 days (p = 0.03) in the time-matched control group.
Conclusions: IV autologous bone marrow-derived mononuclear cell therapy is associated with lower treatment intensity required to manage intracranial pressure, associated severity of organ injury, and duration of neurointensive care following severe traumatic brain injury. This may corroborate preclinical data that autologous bone marrow-derived mononuclear cell therapy attenuates the effects of inflammation in the early post-traumatic brain injury period.
Figures
Comment in
-
Cell-based therapy for pediatric traumatic brain injury: not (yet) an update to the traumatic brain injury guidelines.Pediatr Crit Care Med. 2015 Mar;16(3):294-5. doi: 10.1097/PCC.0000000000000347. Pediatr Crit Care Med. 2015. PMID: 25738929 Free PMC article. No abstract available.
References
-
- Walker PA, Harting MT, Shah SK, Cox CS. Current trends in cell therapy for pediatric acquired brain injury. Minerva pediatrica. 2010 Feb;62(1):91–106. - PubMed
-
- Bowman SM, Bird TM, Aitken ME, Tilford JM. Trends in hospitalizations associated with pediatric traumatic brain injuries. Pediatrics. 2008 Nov;122(5):988–993. - PubMed
-
- Mustafa AG, Alshboul OA. Pathophysiology of traumatic brain injury. Neurosciences (Riyadh) 2013 Jul;18(3):222–234. - PubMed
-
- Hetz RA, Bedi SS, Olson S, Olsen A, Cox CS., Jr. Progenitor cells: therapeutic targets after traumatic brain injury. Translational stroke research. 2012 Sep;3(3):318–323. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

