Correlates and Escitalopram Treatment Effects on Sleep Disturbance in Patients with Acute Coronary Syndrome: K-DEPACS and EsDEPACS
- PMID: 25581916
- PMCID: PMC4481005
- DOI: 10.5665/sleep.4822
Correlates and Escitalopram Treatment Effects on Sleep Disturbance in Patients with Acute Coronary Syndrome: K-DEPACS and EsDEPACS
Abstract
Study objectives: To investigate the correlates of sleep disturbance and to assess escitalopram treatment effects of depression on sleep disturbance in patients with acute coronary syndrome (ACS).
Design: A cross-sectional study in patients with ACS within 2 w post-ACS, and a 24-w double-blind controlled trial of escitalopram against placebo for patients with ACS who have comorbid depressive disorders.
Setting: A university hospital in South Korea.
Participants: There were 1,152 patients with ACS who were consecutively recruited. Of 446 patients with comorbid depressive disorders, 300 were randomized to the trial.
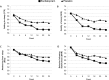
Measurements and results: Sleep disturbance was evaluated by the Leeds Sleep Evaluation Questionnaire. Demographic and clinical characteristics were assessed, including cardiovascular risk factors, current cardiac status, and depressive symptoms. Depressive symptoms were most strongly and consistently associated with sleep disturbance. In addition, older age, female sex, hypertension, and more severe ACS status were associated with certain aspects of sleep disturbance. Escitalopram was significantly superior to placebo for improving sleep disturbance over the 24-w treatment period. These effects were substantially explained by improvement in depressive symptoms.
Conclusions: Depression screening is indicated in patients with acute coronary syndrome with sleep disturbance. Successful treatment of depression has beneficial effects on sleep outcomes in these patients.
Clinical trials information: ClinicalTrial.gov identifier for the 24-w drug trial, NCT00419471.
Keywords: acute coronary syndrome; depression; double-blind study; escitalopram; sleep disorders.
© 2015 Associated Professional Sleep Societies, LLC.
Figures
Comment in
-
Mend the Mind and Mind the "MCC".Sleep. 2015 Jul 1;38(7):1001-3. doi: 10.5665/sleep.4794. Sleep. 2015. PMID: 26085292 Free PMC article. No abstract available.
References
-
- Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. - PubMed
-
- Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. - PubMed
-
- Coryell VT, Ziegelstein RC, Hirt K, Quain A, Marine JE, Smith MT. Clinical correlates of insomnia in patients with acute coronary syndrome. Int Heart J. 2013;54:258–65. - PubMed
-
- Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229–35. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

