MicroRNA-1 aggravates cardiac oxidative stress by post-transcriptional modification of the antioxidant network
- PMID: 25583113
- PMCID: PMC4406930
- DOI: 10.1007/s12192-014-0565-9
MicroRNA-1 aggravates cardiac oxidative stress by post-transcriptional modification of the antioxidant network
Abstract
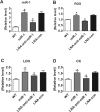
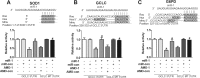
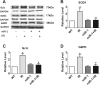
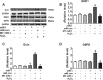
Oxidative stress plays an important role in cardiovascular diseases. Studies have shown that miR-1 plays an important role in the regulation of cardiomyocyte apoptosis, which can be the result of oxidative stress. This study was designed to determine whether increased miR-1 levels lead to alterations in the expression of proteins related to oxidative stress, which could contribute to heart dysfunction. We compared cardiac function in wild-type (WT) and miR-1 transgene (miR-1/Tg) C57BL/6 mice (n ≥ 10/group). Echocardiography showed that stroke volume (SV), ejection fraction (EF), and fractional shortening (FS) were significantly decreased in miR-1/Tg mice. Concomitantly, the level of reactive oxygen species (ROS) was elevated in the cardiomyocytes from the miR-1/Tg mice, and activities of lactate dehydrogenase (LDH) and creatinine kinase (CK) in plasma were also increased in the miR-1/Tg mice. All of these changes could be reversed by LNA-anti-miR-1. In the cardiomyocytes of neonatal Wistar rats, overexpression of miR-1 exhibits higher ROS levels and lower resistance to H2O2-induced oxidative stress. We demonstrated that SOD1, Gclc, and G6PD are novel targets of miR-1 for post-transcriptional repression. MicroRNA-1 post-transcriptionally represses the expression of SOD1, Gclc, and G6PD, which is likely to contribute to the increased ROS level and the susceptibility to oxidative stress of the hearts of miR-1 transgenic mice.
Figures

References
-
- Cheng X, Siow RC, Mann GE. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor-E2-related factor 2-Kelch-like ECH-associated protein 1 defense pathway. Antioxid Redox Signal. 2011;14(3):469–487. doi: 10.1089/ars.2010.3283. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

